Organic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seminar 1 1. Classification and Nomenclature of Organic Compounds
Seminar_1 1. Classification and nomenclature of organic compounds. 2. Reaction mechanism 3. Saturated hydrocarbons (alkanes and cycloalkanes). TEST - Alkanes and Cycloalkanes 1. Classification and nomenclature of organic compounds. General Classification of Organic compounds Classification by functional group Base Name or Hydrocarbon name Chain Primary suffix Generic name C–C –ane Alkane C=C –ene Alkene CºC –yne Alkyne If the parent chain contains two, three or more double or triple bonds, then the following suffixes are used : Bond Two Three Double bond –diene –triene Triple bond –diyne –triyne The root word and the primary suffix together are known as Base Name or Hydrocarbon name. Formula IUPAC name( Base name) CH 4 Methane (Meth+ane) CH 3(CH 2)3CH 3 Pentane (Pent+ane) CH 3(CH 2)23 CH 3 Pentacosane (Pentacos+ane) CH 2 = CH 2 Ethene (Eth+ene) CH 3CH 2CH = CH 2 Butene (But + ene) CH 3CH 2CH=CH 2 Butene (But+ene) CH 3(CH 2)12 CH=CH 2 Pentadecene (Pentadec+ene) CH ≡CH Ethyne (Eth+yne) CH 3(CH 2)6C≡CH Nonyne (Non+yne) CH 3C≡CH Propyne (Prop+yne) 2. Reaction mechanism CATEGORIES OF ORGANIC REACTIONS Virtually all organic reactions fall into one of four categories: They are either substitutions, additions (cycloaddition), eliminations, or rearrangements, Ox/Red . (S) Substitutions are the characteristic reactions of saturated compounds such as alkanes and alkyl halides, and of aromatic compounds (even though they are unsaturated). In a substitution, one group replaces another. For example, methyl chloride reacts with sodiumhydroxide to produce methyl alcohol and sodium chloride: In this reaction a hydroxide ion from sodium hydroxide replaces the chlorine of methyl chloride. -

Mechanisms 1) Free Radical Substitution – Alkane
Mechanisms 1) Free radical substitution – Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion • UV light breaks the C – Cl bond releasing chlorine radical . CFCl3F à CCl2F + Cl • This chlorine radical catalyses the decomposition of ozone with the chlorine radical coming out unchanged (and available for more ozone decomposition). Cl + O3 à ClO + O2 . ClO + O3 à Cl + 2O2 Overall 2O3(g) à 3O2(g) 3) Nucleophilic substitution of halogenoalkanes a) With aqueous hydroxide, OH- Hydrolysis – forming alcohols • This reaction converts a halogenoalkane to an alcohol Reagents: Aqueous sodium hydroxide Conditions: Reflux Hydrolysis: Splitting a molecule apart by using water molecules b) With ethanolic potassium cyanide, KCN – forming nitriles • This reaction converts a halogenoalkane to an alkyl nitrile • This is a key reaction in chemical synthesis as the carbon chain length is increased Reagents: Potassium cyanide dissolved in ethanol Conditions: Reflux c) With excess ethanolic ammonia, NH3 – forming amines • This reaction converts a halogenoalkane to amines Reagents: Excess ethanolic ammonia Conditions: Reflux The Mechanism 4) Elimination of halogenoalkanes With ethanolic potassium hydroxide, reflux – forming alkenes Reagents: KOH dissolved in ethanol Conditions: Reflux Substitution vs elimination Substitution Elimination Aqueous conditions – substitution Ethanolic conditions – Elimination predominates predominates OH- behaves as a nucleophile OH- behaves as a base (accepting a proton) 50 : 50 mixture of water -

Organic Chemistry-II (Reaction Mechanism-1) MODULE No
____________________________________________________________________________________________________ Subject Chemistry Paper No and Title Paper 5: Organic Chemistry-II (Reaction Mechanism-I) Module No and Title Module 1: Types of Organic Reactions Module Tag CHE_P5_M1_e-Text CHEMISTRY PAPER No. 5: Organic Chemistry-II (Reaction Mechanism-1) MODULE No. 1: Types of Organic Reactions ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Types of Organic Reactions 3.1 Substitution Reactions 3.2 Addition Reactions 3.3 Elimination Reactions 3.4. Rearrangement Reactions 3.5 Oxidation and Reduction Reactions 4. Summary CHEMISTRY PAPER No. 5: Organic Chemistry-II (Reaction Mechanism-1) MODULE No. 1: Types of Organic Reactions ____________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to • Know the various types of organic reactions • Learn the difference between each type of reaction • Identify the reaction types depending upon reactants and conditions • Analyze organic reactions 2. Introduction An organic reaction is a change in structure or functional group leading to formation of a new substance. The compound undergoing a change in structure or functional group is called a reactant or substrate. The knowledge of organic reactions helps in the synthesis of useful chemical compounds such as polymers, dyes, drugs, perfumes, cosmetics, -

Radical Pathway for Reductive Dehalogenation and Nucleophilic Substitution of Hetaryl Halides
A RADICAL PATHWAY FOR REDUCTIVE DEHALOGENATION AND NUCLEOPHILIC SUBSTITUTION OF HETARYL HALIDES By TERENCE MILLER OESTREICH A DISSERTATION PRESENTED TO THE GRADUATE COUNCIL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1973 To My Wife, Martha ACKNOWLEDGEMENT The author will always be indebted to Dr. John A. Zoltewicz, Chairman of his Supervisory Committee, for his perceptive guidance and patient support during the course of this research. Appreciation is also extended to the other members of his Committee: Dr. Merle A. Battiste, Dr. Richard D. Dresdner, Dr. Paul Tarrant, and Dr. Robert B. Bennett. Special gratitude is due his wife, Martha, for her unfailing support and understanding during the disap- pointments and achievements of these years. The friendship and assistance of other members of his research group will always be remembered. Thanks are extended to his wife, Martha, and Mrs. Judi Nielsen for their help in preparing the manuscript, Financial support from the Chemistry Department of the University of Florida and from the National Science Foundation is gratefully acknowledged. TABLE OF CONTENTS Page ACKNOWLEDGEMENT iii LIST OF TABLES vi LIST OF FIGURES viii ABSTRACT xi CHAPTER 1 INTRODUCTION 1 2. ALKOXIDE ION PROMOTED REDUCTIVE DEHALOGENATION OF HETARYL HAL IDES 5 Results 5 Discussion 37 3. ALKOXIDE ION PROMOTED NUCLEOPHILIC SUBSTITUTION OF HETARYL HALIDES 52 Results 52 Discussion 80 4. AMIDE ION PROMOTED NUCLEOPHILIC SUBSTITUTION OF 4-HALOISOQUINOLINES 91 Results 91 Discussion 104 5. COVALENT AMINATION AND ANIONIC SIGMA COMPLEXES OF ISOQUINOLINE DERIVATIVES 110 Results 110 Discussion 118 6. -

Free Radical and Ionic Reactions of (Benzoylmethyl)Mercurials Shekhar V
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1991 Free radical and ionic reactions of (Benzoylmethyl)mercurials Shekhar V. Kulkarni Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Kulkarni, Shekhar V., "Free radical and ionic reactions of (Benzoylmethyl)mercurials " (1991). Retrospective Theses and Dissertations. 10048. https://lib.dr.iastate.edu/rtd/10048 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfihn master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

CHM251 Organic Chemistry I Feb 2014
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs: February 26, 2014 Effective: Fall 2014 1. Course Number: CHM251 / CHM251L Course Title: Organic Chemistry I / Organic Chemistry I Laboratory 2. Description: This course covers organic nomenclature, bonding, structure, reaction theory, aliphatic hydrocarbons, functional groups, stereochemistry, aromatic hydrocarbons, alkyl halides, and reaction mechanisms. The laboratory emphasizes basic laboratory techniques for separation, purification and synthesis. (3 class hours/ 4 laboratory hours) 3. Student Learning Outcomes: (instructional objectives; intellectual skills): Upon successful completion of this course, students are able to do the following. • Draw and interpret Lewis, condensed, and line-angle structural formulas and show which atoms bare formal changes • Draw resonance forms and use them to predict stabilities • Calculate empirical and molecular formulas from elemental compositions • Predict relative acidities and basicities based on structure, bonding and resonance of conjugate acid- base pairs • Identify nucleophiles (Lewis bases) and electrophiles (Lewis acids), and write reactions for Lewis acid- base reactions • Draw the structure of a singe bond, a double bond and a triple bond. • Predict the hybridization and geometry of the atoms in a molecule • Draw three dimensional representation of a given molecule • Identify constitutional -

Free Radical Reactions of Allylic and Propargylic Derivatives Yuh-Wern Wu Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1990 Free radical reactions of allylic and propargylic derivatives Yuh-Wern Wu Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Wu, Yuh-Wern, "Free radical reactions of allylic and propargylic derivatives " (1990). Retrospective Theses and Dissertations. 9473. https://lib.dr.iastate.edu/rtd/9473 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Advanced Higher Chemistry Unit 3
Advanced Higher Unit 3- Organic Chemistry Advanced Higher Chemistry Unit 3 - Organic Chemistry PERMEATING ASPECTS & REACTION MECHANISMS Learning Outcomes Questions & Answers KHS Chemistry Jan 2007 page 1 Permeating Aspects & Reaction Mechanisms Advanced Higher Unit 3- Organic Chemistry 1. PERMEATING ASPECTS (Of Organic Chemistry) Reaction Types 3.1.1 Equations can be written for the following reaction types and, given equations, these reaction types can be identified. substitution addition elimination condensation hydrolysis oxidation reduction Reaction Mechanisms 3.1.2 The following reaction mechanisms can be described in terms of electron shifts. ( i) radical substitution of alkanes ( ii) electrophilic addition to alkenes carbocation mechanism cyclic ion intermediate mechanism (iii) nucleophilic substitution SN1 and SN2 Hydrocarbons & Haloalkanes 3.2.4 Alkanes undergo substitution reactions with chlorine and bromine by a chain reaction mechanism. 3.2.5 The chain reaction includes the following steps ( i) initiation by homolytic fission to produce radicals ( ii) propagation (iii) termination 3.2.7 Alkenes can be prepared in the laboratory by ( i) dehydration of alcohols using aluminium oxide, concentrated sulphuric acid or orthophosphoric acid (ii) base-induced elimination of hydrogen halides from monohalogenoalkanes. 3.2.8 Alkenes undergo: ( i) catalytic addition with hydrogen to form alkanes ( ii) addition with halogens to form dihalogenoalkanes (iii) addition with hydrogen halides according to Markownikoff’s rule to form monohalogenoalkanes ( iv) acid-catalysed addition with water according to Markownikoff’s rule to form alcohols. 3.2.9 The mechanisms of the above reactions involve ( i) for halogenation cyclic ion intermedate ( ii) for hydrohalogenation carbocation intermediate (iii) for acid catalysed hydration carbocation intermediate KHS Chemistry Jan 2007 page 2 Permeating Aspects & Reaction Mechanisms Advanced Higher Unit 3- Organic Chemistry 3.2.12 Monohalogenoalkanes undergo nucleophilic substitution reactions. -

Regioselectivity in Free Radical Bromination of Unsymmetrical Dimethylated Pyridines
ABSTRACT REGIOSELECTIVITY IN FREE RADICAL BROMINATION OF UNSYMMETRICAL DIMETHYLATED PYRIDINES by Rajesh Thapa During a literature review some curious inconsistencies in the free radical bromination of picolines were noted. To achieve a better understanding of the mechanisms and regioselectivity we reran these reactions, extending our work to unsymmetrical lutidines using N-bromosuccinimide in limiting amount. Characterization of the products was done with GC/MS and H NMR. The regioselectivity of bromination in unsymmetrical dimethylpyridines shows that nitrogen in the ring is deactivating inductively. The competition between 2,3, 2,4 and 2,5 dimethyl pyridine towards bromination results with bromination in the methyl group farthest from the N in the ring. 3,4-Lutidine shows only the 4,4- dibrominated product. REGIOSELECTIVITY IN FREE RADICAL BROMINATION OF UNSYMMETRICAL DIMETHYLATED PYRIDINES A Thesis Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of Chemistry and Biochemistry by Rajesh Thapa Miami University Oxford, Ohio 2009 Chair____________________________ Dr. Gilbert Ellery Pacey Advisor ____________________________ Dr. Richard T. Taylor Reader____________________________ Dr. Benjamin W. Gung Reader____________________________ Dr. Hong Wang TABLE OF CONTENTS Page Chapter 1: Review of NBS Bromination 1. Introduction 1 1.1: NBS Bromination 1 1.2: Bromination of aliphatic and alicyclic olefins 2 1.3: Allylic or Benzylic Bromination 3 1.4: Unsaturation -

Organic Chemistry I
6: Organic Chemistry I 6A. Introduction to Organic Chemistry Basic definitions Hydrocarbon is a compound consisting of hydrogen and carbon only to know Saturated: Contain single carbon-carbon bonds only Unsaturated : Contains a C=C double bond Molecular formula: The formula which shows the actual number of each type of atom Empirical formula: shows the simplest whole number ratio of atoms of each element in the compound General formula: algebraic formula for a homologous series e.g. CnH2n Structural formula shows the minimal detail that shows the arrangement of atoms in a molecule, eg for butane: CH3CH2CH2CH3 or CH3(CH2)2CH3, Displayed formula: show all the covalent bonds present in a molecule Drawing Displayed formulae Remember that the shape around the carbon atom in saturated hydrocarbons is H H H H When drawing organic tetrahedral and the bond angle is 109.5o H C C C C H compounds add the hydrogen atoms so that H H H H H H C H each carbon has 4 bonds H C C H H H H Skeletal formula shows the simplified organic formula, shown by removing hydrogen atoms from alkyl chains, leaving just a carbon skeleton and associated functional Groups. OH But-2-ene Butan-1-ol 2-methylbutane cyclohexane cyclohexene N Goalby chemrevise.org 1 Homologous series are families of organic compounds with the same functional group and same general formula. •They show a gradual change in physical properties (e.g. boiling point). • Each member differs by CH2 from the last. • same chemical properties. Functional group is an atom or group of atoms which when present -

Classification of Chemical Reactions. Reactivity of Alkanes, Alkenes
МІНІСТЕРСТВО ОХОРОНИ ЗДОРОВ’Я УКРАЇНИ ХАРКІВСЬКИЙ НАЦІОНАЛЬНИЙ МЕДИЧНИЙ УНІВЕРСИТЕТ CLASSIFICATION OF CH EMICAL REACTIONS. REACTIVITY OF ALKANE S, ALKENES, ARENES, ALCOHOLS AND PHENOLS Methodical instructions for 1 st year students’ self - work in Biological and Bioorganic Chemistry КЛАСИФІКАЦІЯ ХІМІЧНИ Х РЕАКЦІЙ. РЕАКЦІЙНА ЗДАТНІСТЬ АЛКАНІВ, А ЛКЕ НІВ, АРЕНІВ, СПИРТІВ ТА ФЕНОЛІВ Методичні вказівки для самостійної роботи студентів 1 - го курсу з біологічної та біоорганічної хімії Затверджено Вченою радою ХНМУ. Протокол № 2 від 22.02. 2018 Харк ів 2018 Classification of chemical reactions. Reactivity of alkanes, alkenes, arenes, alcohols and phenols : Methodical instructions for the 1 st - year students’ self - work in biological and bioorganic chemistry (module 1) / compiled by A . O . Syrovaya , V . N . Petyunina , V . O . Makarov et al. – 2 nd edition, revised, corrected and expanded – Kharkiv : KhNMU , 201 8 . – 24 p . Compiled by: A.O. Syrovaya, V.N. Petyunina, V . O . Makarov , S . V . Andreeva , L . V . Lukianova , S . N . Kozub , T . S . Tishakova , O . L . Levashova , E . V . Savelieva , N . N . Chalenko , O . S . Kalinenko , O.A. Zavada , N.V. Kopoteva, M . A . Vodolazhenko , H.A. Chistiakova Класифікація хімічних реакцій. реакцій. Реакційна здатність алканів, алкенів, аренів, спиртів та фенолів : метод. вказ. для студентів 1 курсу / у клад. Сирова Г.О., Петюніна В.М., Макаров В.О. та ін. – 2 - е вид., переробл., випр., доп. – Харків: ХНМУ , 2018. – 24 с. У кладачі : Г.О. Сирова, В.М. Петюніна, В.О. Макаров, С.В. Андрєєва, Л.В. Лук’янова, С.М. Козуб , Т.С. Тішакова , О.Л. Левашова , О.В. Савельєва , Н.М. Чаленко, О.С. Каліненко, О.О. -
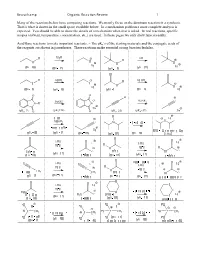
Beauchamp Organic Reaction Review 1
Beauchamp Organic Reaction Review 1 Many of the reactions below have competing reactions. We mostly focus on the dominant reaction in a synthesis. That is what is shown in the small space available below. In a mechanism problem a more complete analysis is expected. You should be able to show the details of a mechanism when that is asked. In real reactions, specific recipes (solvent, temperature, concentration, etc.) are used. In these pages we only show typical results. Acid/Base reactions to make important reactants. – The pKa’s of the starting materials and the conjugate acids of the reagents are shown in parentheses. These reactions make essential strong base/nucleohiles. O O H NaOH NaNR2 N H N Na (pKa=9) (pKa=16) O (pKa=25) (pKa=37) Na O Beauchamp Organic Reaction Review 2 Free radical substitution reactions at sp3 C-H to make R-Br from alkanes. Br Br2 Br Br2 CH4 h H3C h Br Br Br2 Br2 h h Br Br Br2 Br2 h h Br Br2 Br Br2 h h Br 2 equivalents 2 equivalents Br Br Br2 Br2 Br h h Br Br 2 equivalents 2 equivalents Br Br Br2 Br2 h h E2 reactions to make alkenes (use one eq. potassium t-butoxide with RBr) and alkynes (use 3 eqs. sodium amide with RBr2, first two = E2, third equivalent = acid/base reaction) K Br K O O Br K K O O Br Br Br Br K K O O Beauchamp Organic Reaction Review 3 Br Br Br 1. excess NaNR2 H H 2.