GCM1 (R-06): Sc-101173
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Stem Cells from Single Blastomeres Reveal Pathways of Embryonic Or Trophoblast Fate Specification Tamara Zdravkovic1,2,3,4,5,‡, Kristopher L
© 2015. Published by The Company of Biologists Ltd | Development (2015) 142, 4010-4025 doi:10.1242/dev.122846 STEM CELLS AND REGENERATION RESEARCH ARTICLE Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification Tamara Zdravkovic1,2,3,4,5,‡, Kristopher L. Nazor6,‡, Nicholas Larocque1,2,3,4,5, Matthew Gormley1,2,3,4,5, Matthew Donne1,2,3,7, Nathan Hunkapillar1,2,3,4,5, Gnanaratnam Giritharan8, Harold S. Bernstein4,9, Grace Wei4,10, Matthias Hebrok10, Xianmin Zeng11, Olga Genbacev1,2,3,4,5, Aras Mattis4,12, Michael T. McMaster4,5,13, Ana Krtolica8,*, Diana Valbuena14, Carlos Simón14, Louise C. Laurent6,15, Jeanne F. Loring6 and Susan J. Fisher1,2,3,4,5,7,§ ABSTRACT INTRODUCTION For many reasons, relatively little is known about human Mechanisms of initial cell fate decisions differ among species. To gain preimplantation development. The small number of cells makes insights into lineage allocation in humans, we derived ten human embryos of any species difficult to study. In humans, the technical embryonic stem cell lines (designated UCSFB1-10) from single difficulties are compounded by other challenges. Genetic variation blastomeres of four 8-cell embryos and one 12-cell embryo from a among individuals could contribute to developmental differences, a single couple. Compared with numerous conventional lines from well-appreciated phenomenon in the mouse (Dackor et al., 2009), blastocysts, they had unique gene expression and DNA methylation which is difficult to assess in humans owing to the limited patterns that were, in part, indicative of trophoblast competence. At a availability of embryos that are donated for research. -

Identification of a Novel Mutation Disrupting the DNA Binding Activity
443 LETTER TO JMG J Med Genet: first published as 10.1136/jmg.2004.026898 on 29 April 2005. Downloaded from Identification of a novel mutation disrupting the DNA binding activity of GCM2 in autosomal recessive familial isolated hypoparathyroidism L Baumber, C Tufarelli, S Patel, P King, C A Johnson, E R Maher, R C Trembath ............................................................................................................................... J Med Genet 2005;42:443–448. doi: 10.1136/jmg.2004.026898 ypoparathyroidism is a heterogeneous group of dis- orders with both acquired and inherited causes, each Key points Hpresenting clinically with hypocalcaemia. Familial cases of hypoparathyroidism may be due to an isolated N Hypoparathyroidism is a heterogeneous group of defect of the parathyroid glands or be a component of a disorders with both acquired and inherited causes, syndrome disorder, examples of which include DiGeorge, each presenting clinically with hypocalcaemia. hypoparathyroidism-retardation-dysmorphism, and Kenny- N Familial isolated hypoparathyroidism is characterised 1 Caffey syndrome. Familial isolated hypoparathyroidism by hypocalcaemia and hyperphosphataemia and may (FIH) is characterised by hypocalcaemia and hyperpho- be due to an inherited deficiency or the abnormal sphataemia and may be due to an inherited deficiency or activity of parathyroid hormone. the abnormal activity of parathyroid hormone (PTH). FIH is heterogeneous with X linked, autosomal dominant, and N Recently, a homozygous deletion within the human autosomal recessive modes of inheritance reported.2 GCM2 gene has been shown to underlie hypopar- Mutations in the PTH gene on chromosome 11p have been athyroidism in one patient, while other compelling described in both autosomal dominant and autosomal evidence indicates a critical role for GCM2 in normal recessive forms of the disorder.34 Mature PTH is generated parathyroid gland function. -
![View Software Version 1.6.6 [51]](https://docslib.b-cdn.net/cover/1758/view-software-version-1-6-6-51-591758.webp)
View Software Version 1.6.6 [51]
BMC Genomics BioMed Central Research article Open Access Phylogenetic and comparative gene expression analysis of barley (Hordeum vulgare) WRKY transcription factor family reveals putatively retained functions between monocots and dicots Elke Mangelsen1, Joachim Kilian2, Kenneth W Berendzen2, Üner H Kolukisaoglu3, Klaus Harter2, Christer Jansson1,4 and Dierk Wanke*2 Address: 1Department of Plant Biology and Forest Genetics, The Swedish University of Agricultural Sciences (SLU), P.O. Box 7080, SE-750 07 Uppsala, Sweden, 2Center of Plant Molecular Biology (ZMBP), University of Tuebingen, Auf der Morgenstelle 1, D-72076 Tübingen, Germany, 3CELISCA (Center for Life Science Automation), Friedrich-Barnewitz-Strasse 8, D-18119 Rostock, Germany and 4Lawrence Berkeley Laboratory, Earth Science Division, 1 Cyclotron Rd., Berkeley, CA 94720, USA Email: Elke Mangelsen - [email protected]; Joachim Kilian - [email protected]; Kenneth W Berendzen - [email protected]; Üner H Kolukisaoglu - [email protected]; Klaus Harter - [email protected]; Christer Jansson - [email protected]; Dierk Wanke* - [email protected] tuebingen.de * Corresponding author Published: 28 April 2008 Received: 20 October 2007 Accepted: 28 April 2008 BMC Genomics 2008, 9:194 doi:10.1186/1471-2164-9-194 This article is available from: http://www.biomedcentral.com/1471-2164/9/194 © 2008 Mangelsen et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: WRKY proteins belong to the WRKY-GCM1 superfamily of zinc finger transcription factors that have been subject to a large plant-specific diversification. -

Aberrant Gcm1 Expression Mediates Wnt/Β-Catenin Pathway Activation in Folate Deficiency Involved in Neural Tube Defects
Li et al. Cell Death and Disease (2021) 12:234 https://doi.org/10.1038/s41419-020-03313-z Cell Death & Disease ARTICLE Open Access Aberrant Gcm1 expression mediates Wnt/ β-catenin pathway activation in folate deficiency involved in neural tube defects Jianting Li1,QiuXie2,JunGao 3, Fang Wang4,YihuaBao4,LihuaWu4,LihongYang5, Zhizhen Liu1,RuiGuo1, Ajab Khan1, Dan Liu1,CaihuaLi6, Jianxin Wu4 and Jun Xie 1 Abstract Wnt signaling plays a major role in early neural development. An aberrant activation in Wnt/β-catenin pathway causes defective anteroposterior patterning, which results in neural tube closure defects (NTDs). Changes in folate metabolism may participate in early embryo fate determination. We have identified that folate deficiency activated Wnt/β-catenin pathway by upregulating a chorion-specific transcription factor Gcm1. Specifically, folate deficiency promoted formation of the Gcm1/β-catenin/T-cell factor (TCF4) complex formation to regulate the Wnt targeted gene transactivation through Wnt-responsive elements. Moreover, the transcription factor Nanog upregulated Gcm1 transcription in mESCs under folate deficiency. Lastly, in NTDs mouse models and low-folate NTDs human brain samples, Gcm1 and Wnt/β-catenin targeted genes related to neural tube closure are specifically overexpressed. These results indicated that low-folate level promoted Wnt/β-catenin signaling via activating Gcm1, and thus leaded into aberrant vertebrate neural development. 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; Introduction embryonic neural stem cells inhibit the proliferation of Neural tube defects (NTDs) are severe birth defects neural progenitor cells and promote their differentiation6. thought to be associated with genetic and environmental Aberrant canonical Wnt/β-catenin pathway signaling factors1, resulted from the failure of neural tube closure leads to defective anteroposterior patterning7. -

GCM1 Antibody - N-Terminal Region Rabbit Polyclonal Antibody Catalog # AI16248
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 GCM1 antibody - N-terminal region Rabbit Polyclonal Antibody Catalog # AI16248 Specification GCM1 antibody - N-terminal region - Product Information Application IHC, WB Primary Accession Q9NP62 Other Accession NM_003643, NP_003634 Reactivity Human, Mouse, Rat, Dog Predicted Human, Mouse, Rat, Dog Host Rabbit Clonality Polyclonal Calculated MW 49kDa KDa Human Lung GCM1 antibody - N-terminal region - Additional Information Gene ID 8521 Alias Symbol GCMA, hGCMa Other Names Chorion-specific transcription factor GCMa, hGCMa, GCM motif protein 1, Glial cells missing homolog 1, GCM1, GCMA Format Liquid. Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and Human Spleen 2% sucrose. Reconstitution & Storage Add 50 ul of distilled water. Final anti-GCM1 antibody concentration is 1 mg/ml in PBS buffer with 2% sucrose. For longer periods of storage, store at 20°C. Avoid repeat freeze-thaw cycles. Precautions GCM1 antibody - N-terminal region is for research use only and not for use in diagnostic or therapeutic procedures. WB Suggested Anti-GCM1 Antibody Titration: 0.2-1 μg/ml GCM1 antibody - N-terminal region - Protein ELISA Titer: 1:7812500 Information Positive Control: Human Placenta Page 1/2 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 Name GCM1 Synonyms GCMA Function Transcription factor involved in the control of expression of placental growth factor (PGF) and other placenta-specific genes (PubMed:<a href="http://www.uniprot.org/c itations/10542267" target="_blank">10542267</a>, PubMed:<a href="http://www.uniprot.org/ci tations/18160678" Human 293T target="_blank">18160678</a>). -

Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications
Stem Cell Rev and Rep DOI 10.1007/s12015-016-9662-8 Detailed Characterization of Human Induced Pluripotent Stem Cells Manufactured for Therapeutic Applications Behnam Ahmadian Baghbaderani 1 & Adhikarla Syama2 & Renuka Sivapatham3 & Ying Pei4 & Odity Mukherjee2 & Thomas Fellner1 & Xianmin Zeng3,4 & Mahendra S. Rao5,6 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract We have recently described manufacturing of hu- help determine which set of tests will be most useful in mon- man induced pluripotent stem cells (iPSC) master cell banks itoring the cells and establishing criteria for discarding a line. (MCB) generated by a clinically compliant process using cord blood as a starting material (Baghbaderani et al. in Stem Cell Keywords Induced pluripotent stem cells . Embryonic stem Reports, 5(4), 647–659, 2015). In this manuscript, we de- cells . Manufacturing . cGMP . Consent . Markers scribe the detailed characterization of the two iPSC clones generated using this process, including whole genome se- quencing (WGS), microarray, and comparative genomic hy- Introduction bridization (aCGH) single nucleotide polymorphism (SNP) analysis. We compare their profiles with a proposed calibra- Induced pluripotent stem cells (iPSCs) are akin to embryonic tion material and with a reporter subclone and lines made by a stem cells (ESC) [2] in their developmental potential, but dif- similar process from different donors. We believe that iPSCs fer from ESC in the starting cell used and the requirement of a are likely to be used to make multiple clinical products. We set of proteins to induce pluripotency [3]. Although function- further believe that the lines used as input material will be used ally identical, iPSCs may differ from ESC in subtle ways, at different sites and, given their immortal status, will be used including in their epigenetic profile, exposure to the environ- for many years or even decades. -

Primate-Specific Mir-515 Family Members Inhibit Key Genes in Human Trophoblast Differentiation and Are Upregulated in Preeclamps
Primate-specific miR-515 family members inhibit key PNAS PLUS genes in human trophoblast differentiation and are upregulated in preeclampsia Ming Zhanga,1, Sribalasubashini Muralimanoharana, Alison C. Wortmanb, and Carole R. Mendelsona,b,2 aDepartment of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390-9038; and bDepartment of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX 75390-9032 Edited by R. Michael Roberts, University of Missouri–Columbia, Columbia, MO, and approved September 29, 2016 (received for review May 20, 2016) Dysregulation of human trophoblast invasion and differentiation can Estrogens formed by placental aromatase may enhance angiogen- result in preeclampsia (PE), a hypertensive disorder of pregnancy with esis, uteroplacental blood flow, and reduce systemic vascular significant morbidity and mortality for mother and offspring. miRNA resistance (10–14). microarray analysis of RNA from human cytotrophoblasts (CytT), In previous studies using transgenic mice and transfected human before and after differentiation to syncytiotrophoblast (SynT) in trophoblasts in primary culture, we identified an ∼300-bp region primary culture, revealed that members of miR-515 family—including upstream of placenta-specific hCYP19A1 exon I.1 that acts as an miR-515-5p, miR-519e-5p, miR-519c-3p, and miR-518f, belonging to enhancesome and is essential for placenta-specific hCYP19A1 the primate- and placenta-specific chromosome 19 miRNA cluster expression. This genomic region contains response elements for es- (C19MC)—were significantly down-regulated upon human SynT dif- trogen receptor-α (ERα) (15), estrogen-related receptor-γ (16), Sp1 ferentiation. The proto-oncogene, c-MYC, which declines during SynT (9), and glial cells missing 1 (GCM1) (17, 18); each of these tran- pri-miR-515-1 differentiation, interacted with E-boxes upstream of scription factors serves a crucial role in placenta-specific hCYP19A1 pri-miR-515-2 and , encoding these mRNAs, to enhance their expres- expression. -

Early Onset Preeclampsia in a Model for Human Placental Trophoblast
Early onset preeclampsia in a model for human placental trophoblast Megan A. Sheridana,b,c,1, Ying Yangb,d,e,1, Ashish Jainf,g, Alex S. Lyonsa,b, Penghua Yangb,h, Sambasiva R. Brahmasanib,i, Aihua Daib, Yuchen Tianb, Mark R. Ellersieckj, Geetu Tutejaf,g, Danny J. Schustk, Laura C. Schulzk, Toshihiko Ezashib,d,1, and R. Michael Robertsa,b,d,2 aDepartment of Biochemistry, University of Missouri, Columbia, MO 65211; bBond Life Sciences Center, University of Missouri, Columbia, MO 65211; cDepartment of Pathology, Centre for Trophoblast Research, University of Cambridge, Cambridge CB2 1QP, United Kingdom; dDivision of Animal Sciences, University of Missouri, Columbia, MO 65211; eDepartment of Molecular Pharmacology and Physiology, University of South Florida, Tampa, FL 33620; fDepartment of Genetics, Development, and Cell Biology, Iowa State University, Ames, IA 50011; gBioinformatics and Computational Biology, Iowa State University, Ames, IA 50011; hDepartment of Obstetrics, Gynecology, and Reproductive Sciences, School of Medicine, University of Maryland, Baltimore, Baltimore, MD 21201; iCentre for Cellular and Molecular Biology (CCMB), Hyderabad 500007, India; jAgriculture Experiment Station, University of Missouri, Columbia, MO 65211; and kDepartment of Obstetrics, Gynecology and Women’s Health, University of Missouri, Columbia, MO 65212 Contributed by R. Michael Roberts, January 8, 2019 (sent for review September 24, 2018; reviewed by Mana M. Parast and Soumen Paul) We describe a model for early onset preeclampsia (EOPE) that uses that provoke endothelial dysfunction and inflammation in the ma- induced pluripotent stem cells (iPSCs) generated from umbilical ternal vessels. In a normal pregnancy, up-regulation of vascular cords of EOPE and control (CTL) pregnancies. -

ADHD) Gene Networks in Children of Both African American and European American Ancestry
G C A T T A C G G C A T genes Article Rare Recurrent Variants in Noncoding Regions Impact Attention-Deficit Hyperactivity Disorder (ADHD) Gene Networks in Children of both African American and European American Ancestry Yichuan Liu 1 , Xiao Chang 1, Hui-Qi Qu 1 , Lifeng Tian 1 , Joseph Glessner 1, Jingchun Qu 1, Dong Li 1, Haijun Qiu 1, Patrick Sleiman 1,2 and Hakon Hakonarson 1,2,3,* 1 Center for Applied Genomics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; [email protected] (Y.L.); [email protected] (X.C.); [email protected] (H.-Q.Q.); [email protected] (L.T.); [email protected] (J.G.); [email protected] (J.Q.); [email protected] (D.L.); [email protected] (H.Q.); [email protected] (P.S.) 2 Division of Human Genetics, Department of Pediatrics, The Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 3 Department of Human Genetics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA * Correspondence: [email protected]; Tel.: +1-267-426-0088 Abstract: Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder with poorly understood molecular mechanisms that results in significant impairment in children. In this study, we sought to assess the role of rare recurrent variants in non-European populations and outside of coding regions. We generated whole genome sequence (WGS) data on 875 individuals, Citation: Liu, Y.; Chang, X.; Qu, including 205 ADHD cases and 670 non-ADHD controls. The cases included 116 African Americans H.-Q.; Tian, L.; Glessner, J.; Qu, J.; Li, (AA) and 89 European Americans (EA), and the controls included 408 AA and 262 EA. -
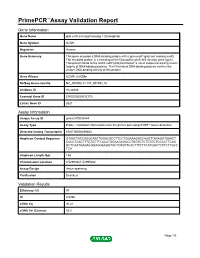
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name glial cells missing homolog 1 (Drosophila) Gene Symbol GCM1 Organism Human Gene Summary This gene encodes a DNA-binding protein with a gcm-motif (glial cell missing motif). The encoded protein is a homolog of the Drosophila glial cells missing gene (gcm). This protein binds to the GCM-motif (A/G)CCCGCAT a novel sequence among known targets of DNA-binding proteins. The N-terminal DNA-binding domain confers the unique DNA-binding activity of this protein. Gene Aliases GCMA, hGCMa RefSeq Accession No. NC_000006.11, NT_007592.15 UniGene ID Hs.28346 Ensembl Gene ID ENSG00000137270 Entrez Gene ID 8521 Assay Information Unique Assay ID qHsaCIP0030444 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000259803 Amplicon Context Sequence GTAACTACCAGGCAATTGGACGCCTTCCTGGAAAGACCAAGTTAAAGGTAAACT CCCCTGACTTTGTGTTTCACCTGGAAGAGACCTGGTCTCTGTGCTCCCCTTCAG GCTCAATGAGACGGAGGAAGGTGCTGTGTTCACTTTCTTCATGGCTCTTCTTGCC TCA Amplicon Length (bp) 136 Chromosome Location 6:52993661-52995682 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 97 R2 0.9996 cDNA Cq 30.21 cDNA Tm (Celsius) 83.5 Page 1/5 PrimePCR™Assay Validation Report gDNA Cq 44.24 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report GCM1, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak -

P45nf-E2 Represses Gcm1 in Trophoblast Cells to Regulate
RESEARCH ARTICLE 2235 Development 138, 2235-2247 (2011) doi:10.1242/dev.059105 © 2011. Published by The Company of Biologists Ltd p45NF-E2 represses Gcm1 in trophoblast cells to regulate syncytium formation, placental vascularization and embryonic growth Muhammed Kashif1, Andrea Hellwig2, Alexandre Kolleker3, Khurrum Shahzad1, Hongjie Wang1,4, Siegfried Lang5, Juliane Wolter1, Madhusudhan Thati1, Ilya Vinnikov1, Angelika Bierhaus1, Peter P. Nawroth1 and Berend Isermann1,*,† SUMMARY Absence of the leucine zipper transcription factor p45NF-E2 results in thrombocytopenia, impaired placental vascularization and intrauterine growth restriction (IUGR) in mice. The mechanism underlying the p45NF-E2-dependent placental defect and IUGR remains unknown. Here, we show that the placental defect and IUGR of p45NF-E2 (Nfe2) null mouse embryos is unrelated to thrombocytopenia, establishing that embryonic platelets and platelet-released mediators are dispensable for placentation. Rather, p45NF-E2, which was hitherto thought to be specific to hematopoietic cells, is expressed in trophoblast cells, where it is required for normal syncytiotrophoblast formation, placental vascularization and embryonic growth. Expression of p45NF-E2 in labyrinthine trophoblast cells colocalizes with that of Gcm1, a transcription factor crucial for syncytiotrophoblast formation. In the absence of p45NF-E2, the width of syncytiotrophoblast layer 2 and the expression of Gcm1 and Gcm1-dependent genes (Synb and Cebpa) are increased. In vitro, p45NF-E2 deficiency results in spontaneous syncytiotrophoblast formation, which can be reversed by Gcm1 knockdown. Increased Gcm1 expression in the absence of p45NF-E2 is dependent on enhanced protein acetylation, including post-translational modification of Gcm1. Increasing and inhibiting acetylation in the placenta of wild-type control embryos phenocopies and corrects, respectively, the changes observed in p45NF-E2-deficient embryos. -

Transcription Factors Underlying the Development and Endocrine Functions of the Placenta † † † JAMES C
Transcription Factors Underlying the Development and Endocrine Functions of the Placenta † † † JAMES C. CROSS,* LYNN ANSON-CARTWRIGHT, AND IAN C. SCOTT *Department of Biochemistry & Molecular Biology, University of Calgary Faculty of Medicine, Calgary, Alberta T2N 4N1 Canada; †Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Ontario M5G 1X5 Canada ABSTRACT The placenta has been the subject of extensive basic research efforts in two distinct fields. The developmental biology of placenta has been studied because it is the first organ to develop during embryogenesis and because a number of different gene mutations in mice result in embryonic lethality due to placental defects. The trophoblast cell lineage is relatively simple such that only two major, terminally differentiated cell types appear: an “invasive trophoblast” cell subtype such as extravillous cytotrophoblast cells in humans and trophoblast giant cells in mice, and a “transport trophoblast” cell subtype that is a syncytium (syncytiotrophoblast) in humans and mice. These two cell types also have been the focus of endocrinologists because they are the source of major placental hormones. Understanding the transcriptional regulation of placental hormone genes has given insights into the control of specificity of gene expression. Because most placental hormones are produced by very specific trophoblast cell subtypes, the transcriptional details promise to give insights into cell-subtype specification. The fields of developmental biology and molecular endocrinology appear to be meeting on this common ground with the recent discovery of key transcription factors. Specifically, the basic helix-loop-helix (bHLH) transcription factor Hand1 is essential for differen- tiation of trophoblast giant cells in mice and also regulates the promoter for the giant cell-specific hormone, placental lactogen I gene (Pl1).