Gemfibrozil May 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ezetimibe: a Novel Selective Cholesterol Absorption Inhibitor by Michele Koder, Pharm.D
OREGON DUR BOARD NEWSLETTER A N E VIDENCE B ASED D RUG T HERAPY R ESOURCE COPYRIGHT 2003 OREGON STATE UNIVERSITY. ALL RIGHTS RESERVED Volume 5, Issue 2 Also available on the web and via e-mail list-serve at February 2003 http://pharmacy.orst.edu/drug_policy/newsletter_email.html Ezetimibe: A novel selective cholesterol absorption inhibitor By Michele Koder, Pharm.D. , OSU College of Pharmacy Ezetimibe (Zetia) is a novel selective cholesterol absorption inhibitor that was approved by the FDA in October 2002. Unlike statins (HMG-CoA reductase inhibitors) and bile acid sequestrants, ezetimibe does not inhibit hepatic cholesterol synthesis or increase bile acid secretion. In contrast, ezetimibe selectively inhibits the uptake of dietary cholesterol from enterocytes in the brush border of the intestinal lumen resulting in a decrease in the delivery of dietary cholesterol to the liver and a subsequent decrease in hepatic cholesterol stores and increased cholesterol clearance from the blood.1 Ezetimibe’s unique action has generated interest in its use in combination with other cholesterol-lowering agents. It is indicated for the treatment of primary hypercholesterolemia as monotherapy and in combination with a statin. Ezetimibe is also approved for homozygous familial hypercholesterolemia and homozygous sitosterolemia. TABLE 1: EZETIMIBE CLINICAL TRIAL SUMMARY Study / Design Population Treatment % Change LDL % Change HDL % Change TG Bays et al3 N=432 EZ 5 mg -15.7 +2.9 MC, R, DB, PC LDL 130-250mg/dl EZ 10 mg -18.5 +3.5 NS 12 wk; Phase II TG -

Lipid Lowering Agents, Cognitive Decline, and Dementia: the Three-City Study
Lipid lowering agents, cognitive decline, and dementia: the three-city study. Marie-Laure Ancelin, Isabelle Carrière, Pascale Barberger-Gateau, Sophie Auriacombe, Olivier Rouaud, Spiros Fourlanos, Claudine Berr, Anne-Marie Dupuy, Karen Ritchie To cite this version: Marie-Laure Ancelin, Isabelle Carrière, Pascale Barberger-Gateau, Sophie Auriacombe, Olivier Rouaud, et al.. Lipid lowering agents, cognitive decline, and dementia: the three-city study.: Lipid Lowering Agents and Cognitive Decline. Journal of Alzheimer’s Disease, IOS Press, 2012, 30 (3), pp.629-37. 10.3233/JAD-2012-120064. inserm-00707350 HAL Id: inserm-00707350 https://www.hal.inserm.fr/inserm-00707350 Submitted on 12 Jun 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Lipid lowering agents, cognitive decline, and dementia: the three-city study Marie-Laure Ancelin 1 * , Isabelle Carrière 1 , Pascale Barberger-Gateau 2 , Sophie Auriacombe 2 , Olivier Rouaud 3 , Spiros Fourlanos 4 , Claudine Berr 1 , Anne-Marie Dupuy 1 5 , Karen Ritchie 1 6 1 Neuropsychiatrie : Recherche Epidémiologique -

FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy by Anthony C
Supplement to Supported by an unrestricted educational grant from Abbott Laboratories March/April 2008 FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy By Anthony C. Keech, MBBS, Msc Epid, FRANZCS, FRACP; and Paul Mitchell, MBBS(Hons), MD, PhD, FRANZCO, FRACS, FRCOphth, FAFPHM This agent’s mechanism of benefit in diabetic retinopathy appears to go beyond its effects on lipid concentration or blood pressure, and this potential mechanism of action operates even when glycemic control and blood pressure levels are within goal. ABSTRACT icant relative reduction was seen of almost one-third in PURPOSE the rate of first laser application for retinopathy after The FIELD (Fenofibrate Intervention and Event an average treatment duration of 5 years with fenofi- Lowering in Diabetes) study sought to investigate brate 200 mg/day. whether long-term lipid-lowering therapy with fenofi- In this report, we detail the effects of fenofibrate brate would reduce macro- and microvascular compli- administration on ophthalmic microvascular compli- cations among patients with type 2 diabetes. We previ- cations and attempt to clarify some of the underlying ously reported that in type 2 diabetes patients with pathologies being addressed among patients undergo- adequate glycemic and blood pressure control, a signif- ing laser treatment. Jointly sponsored by The Dulaney Foundation and Retina Today MARCH/APRIL 2008 I SUPPLEMENT TO RETINA TODAY I 1 FIELD Study Revealed Fenofibrate Reduced Need for Laser Treatment for Diabetic Retinopathy Jointly sponsored by The Dulaney Foundation and Retina Today. Release date: April 2008. Expiration date: April 2009. This continuing medical education activity is supported by an unrestricted educational grant from Abbott Laboratories. -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -

The Effects of Statin and Fibrate Drugs on Cholesterol Metabolism And
The effects of statin and fibrate drugs on cholesterol metabolism and steroid production in two fish species. By Aziz Al-Habsi Thesis submitted to the Faculty of Graduate and Postdoctoral Studies University of Ottawa In partial fulfillment of the requirements for the PhD degree in the Ottawa-Carleton Institute of Biology Thèse soumise à la Faculté des Études Supérieurs et Postdoctorales Université d’Ottawa En vue de la réalisation partielle du doctorat à L’Institut de Biologie Ottawa-Carleton ©Aziz Al-Habsi, Ottawa, Canada, 2014 This thesis is dedicated to my wife and children who have always stood by me and dealt with all my absence from many family occasions with a smile. ii Acknowledgments Completing a PhD is truly a marathon event, and I would not have able to complete this journey without the aid and support of countless people over the past seven years. First and foremost I would like to gratefully and sincerely thank my supervisor Dr. Thomas W. Moon for his guidance, understanding, friendship, and most importantly, his patience during my graduate studies at University of Ottawa. His mentorship was paramount in providing a well-rounded experience consistent my long-term career goals. He encouraged me not only to be a biologist but also be an instructor and independent thinker. I am not sure many graduate students are given the opportunity to develop their own individuality and self- sufficiency by being allowed to work with such independence. For everything you’ve done for me, Dr. Moon, I thank you. I would like to extend my thanks the Department of Biology at University of Ottawa, especially those members of my doctoral committee for their input, valuable discussions and accessibility. -

Bempedoic Acid and Inclisiran for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value
Bempedoic Acid and Inclisiran for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value Draft Evidence Report November 12, 2020 Prepared for ©Institute for Clinical and Economic Review, 2020 ICER Staff and Consultants Modeling Team Grace A. Lin, MD, MAS Dhruv S. Kazi, MD, MSc, MS Associate Professor of Medicine and Health Policy Associate Director, Smith Center for Outcomes University of California, San Francisco Research in Cardiology Director, Cardiac Critical Care Unit Jane Jih, MD, MPH Beth Israel Deaconess Medical Center Assistant Professor, Division of General Internal Associate Professor, Harvard Medical School Medicine University of California, San Francisco Foluso Agboola, MBBS, MPH Director, Evidence Synthesis Dr. Kazi was responsible for the development of the ICER cost-effectiveness model, interpretation of results, and drafting of the economic sections of this report; the Rick Chapman, PhD, MS resulting ICER reports do not necessarily represent the Director of Health Economics views of Beth Israel Deaconess Medical Center or ICER Harvard Medical School. Steven D. Pearson, MD, MSc President ICER DATE OF PUBLICATION: November 12, 2020 How to cite this document: Lin GA, Kazi DS, Jih J, Agboola F, Chapman R, Pearson SD. Inclisiran and Bempedoic Acid for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value; Draft Evidence Report. Institute for Clinical and Economic Review, November 12, 2020. https://icer-review.org/material/high-cholesterol-update- draft-evidence-report/ Grace Lin served as the lead author for the report and wrote the background, other benefits, and contextual considerations sections of the report, with Jane Jih serving as a co-author. -
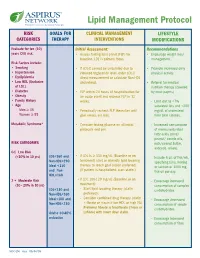
Lipid Management Protocol
Lipid Management Protocol RISK GOALS FOR CLINICAL MANAGEMENT LIFESTYLE CATEGORIES THERAPY INTERVENTIONS MODIFICATIONS Evaluate for ten (10) Initial Assessment: Recommendations years CVD risk. • Assess fasting lipid panel (FLP) for • Encourage weight loss/ baseline. LDL is primary focus. management. Risk Factors include: • Smoking • If LDL-C cannot be calculated due to • Promote increased daily • Hypertension elevated triglyceride level, order LDL-C physical activity. • Dyslipidemia direct measurement or calculate Non-HDL • Low HDL (Exclusive cholesterol. • Referral for medical of LDL) nutrition therapy (covered • Diabetes • FLP within 24 hours of hospitalization for by most payers) • Obesity an acute event and recheck FLP in 12 • Family History weeks. - Limit diet to <7% • Age saturated fats and <200 Men ≥ 45 • Periodically recheck FLP thereafter until mg/dL of cholesterol Women ≥ 55 goal values are met. from total calories. Metabolic Syndrome* • Consider fasting glucose on all initial - Increased consumption protocols and prn. of monounsaturated fatty acids (olive/ peanut/ canola oils, RISK CATEGORIES nuts/peanut butter, avocado, olives). 0-1 Low Risk (<10% in 10 yrs) LDL<160 and • If LDL is ≥ 130 mg/dL (Baseline or on - Include 6 oz. of fish/wk, Non-HDL<190 treatment) start or intensify lipid lowering specifying tuna, herring Ideal <130 therapy to reach goal (statin preferred). or salmon or 1000 mg and Non- (If patient is hospitalized, start statin.) fish oil per day. HDL<160 2 + Moderate Risk • If LDL 100-129 mg/dL (Baseline or on - Encourage increased (10 - 20% in 10 yrs) treatment): consumption of complex LDL<130 and - Start lipid lowering therapy (statin carbohydrates. Non-HDL<160 preferred). -

Product Monograph
PRODUCT MONOGRAPH Pr AA-FENO-MICRO Fenofibrate Capsules 67 mg and 200 mg fenofibrate, micronized formulation House Standard Pr FENOFIBRATE Fenofibrate Capsules 100 mg fenofibrate, non-micronized formulation House Standard Lipid Metabolism Regulator AA PHARMA INC. Date of Preparation: 1165 Creditstone Road Unit #1 October 08, 2019 Vaughan, Ontario L4K 4N7 Control No.: 230394 PRODUCT MONOGRAPH Pr AA-FENO-MICRO Fenofibrate Capsules 67 mg and 200 mg fenofibrate, micronized formulation House Standard Pr FENOFIBRATE Fenofibrate Capsules 100 mg fenofibrate, non-micronized formulation House Standard THERAPEUTIC CLASSIFICATION Lipid Metabolism Regulator ACTIONS AND CLINICAL PHARMACOLOGY Fenofibrate lowers elevated serum lipids by decreasing the low-density lipoprotein (LDL) fraction rich in cholesterol and the very low density lipoprotein (VLDL) fraction rich in triglycerides. In addition, fenofibrate increases the high density lipoprotein (HDL) cholesterol fraction. Fenofibrate appears to have a greater depressant effect on the VLDL than on the low density lipoproteins (LDL). Therapeutic doses of fenofibrate produce elevations of HDL cholesterol, a reduction in the content of the low density lipoproteins cholesterol, and a substantial reduction in the triglyceride content of VLDL. The mechanism of action of fenofibrate has not been definitively established. Work carried out to date suggests that fenofibrate: · enhances the liver elimination of cholesterol as bile salts; · inhibits the biosynthesis of triglycerides and enhances the catabolism of VLDL by increasing the activity of lipoprotein lipase; · has an inhibitory effect on the biosynthesis of cholesterol by modulating the activity of HMG- CoA reductase. Metabolism and Excretion After oral administration with food, fenofibrate is rapidly hydrolysed to fenofibric acid, the active metabolite. -

Fenofibrate Capsules Apotex Standard 67 Mg and 200 Mg
PRODUCT MONOGRAPH PrAPO-FENO-MICRO Fenofibrate Capsules Apotex Standard 67 mg and 200 mg PrAPO-FENOFIBRATE Fenofibrate Capsules Apotex Standard 100 mg Lipid Metabolism Regulator APOTEX INC. 150 Signet Drive Toronto, Ontario DATE OF REVISION: M9L 1T9 October 7, 2014 Control No.: 169773 - 1 - PRODUCT MONOGRAPH PrAPO-FENO-MICRO Fenofibrate Capsules Apotex Standard 67 mg and 200 mg PrAPO-FENOFIBRATE Fenofibrate Capsules Apotex Standard 100 mg THERAPEUTIC CLASSIFICATION Lipid Metabolism Regulator ACTIONS AND CLINICAL PHARMACOLOGY Fenofibrate lowers elevated serum lipids by decreasing the low-density lipoprotein (LDL) fraction rich in cholesterol and the very low density lipoprotein (VLDL) fraction rich in triglycerides. In addition, fenofibrate increases the high density lipoprotein (HDL) cholesterol fraction. Fenofibrate appears to have a greater depressant effect on the VLDL than on the low density lipoproteins (LDL). Therapeutic doses of fenofibrate produce elevations of HDL cholesterol, a reduction in the content of the low density lipoproteins cholesterol, and a substantial reduction in the triglyceride content of VLDL. The mechanism of action of fenofibrate has not been definitively established. Work carried out to date suggests that fenofibrate: · enhances the liver elimination of cholesterol as bile salts; · inhibits the biosynthesis of triglycerides and enhances the catabolism of VLDL by increasing the activity of lipoprotein lipase; · has an inhibitory effect on the biosynthesis of cholesterol by modulating the activity of HMG- CoA reductase. Metabolism and Excretion After oral administration with food, fenofibrate is rapidly hydrolyzed to fenofibric acid, the active metabolite. In man it is mainly excreted through the kidney. Half-life is about 20 hours. In patients with severe renal failure, significant accumulation was observed with a large increase in half-life. -

1 Lipid Lowering Agents, Cognitive Decline, and Dementia
Lipid Lowering Agents, Cognitive Decline, and Dementia: The Three-City Study Marie-Laure Ancelin, PhD.a,b,§,*, Isabelle Carrière, PhD.a,b,§, Pascale Barberger-Gateau PhD.c, Sophie Auriacombe, MDc, Olivier Rouaud, MDd, Spiros Fourlanos, MD, PhD.e, Claudine Berr, PhD.a,b, Anne-Marie Dupuy, PhD.a,b,f, Karen Ritchie, PhD.a,b,g Running title: Lipid Lowering Agents and Cognitive Decline a Inserm, U1061, Montpellier, F-34093 France b Université Montpellier 1, Montpellier, France c Inserm, U897, Bordeaux, F-33076 France d Centre Mémoire Ressources et Recherche, Centre Hospitalier Universitaire, Dijon, France e Department of Diabetes & Endocrinology, Royal Melbourne Hospital, Melbourne, Australia f CHU Montpellier, Laboratoire de Biochimie, Hopital Lapeyronie, Montpellier, France g Faculty of Medicine, Imperial College, London, W12 0NN, U.K. *Corresponding author: Inserm U1061, Hopital La Colombiere, 39, avenue C. Flahault, BP 34493, 34093 Montpellier Cedex 5, France Tel: +33 499 614 562; Fax: +33 499 614 579; E-mail address: [email protected] § Joint first authors Declaration of interest: No conflict of interest. Paper length: 2863 words Title character count: 67 Abstract: 231 words 3 Tables 1 Abstract The aim of this prospective cohort study was to evaluate the effects of lipid lowering agent (LLA) intake on cognitive function in 6830 community-dwelling elderly persons. Cognitive performance (global cognitive functioning, visual memory, verbal fluency, psychomotor speed and executive function), clinical diagnosis of dementia, and fibrate and statin use, were evaluated at baseline, and 2, 4, and 7 year follow-up. Multivariate Cox models were stratified by gender and adjusted for sociodemographic characteristics, mental and physical health including vascular risk factors, and genetic vulnerability (apolipoprotein E and cholesteryl ester transfer protein).