Changes in Food Web Structure Under the Influence of Increased Anthropogenic Nitrogen Inputs to Estuaries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coastal Resources Element 2.2
Coastal Resources Element 2.2 Inventory The Coastal Resources inventory identifies and summarizes a variety of Falmouth’s beaches, coastal banks, shellfish beds, commercial waterfronts, harbors, access points and coastal facilities. Detailed and specific coastal resource information, such as the Shellfish Management Plan and The Future of Falmouth’s South Shore, are found in various Town Hall departments. Shellfish Beds & Habitat Areas The majority of Falmouth’s tidal ponds and rivers are seasonally approved for the harvest of shellfish from November thru April on an annual basis, with each water-body having various quantities of quality shellfish habitat. Areas open for year-round harvest vary from year to year and are more limited in scope, but typically consist of locations in Waquoit Bay, Bournes Pond, Little Harbor (Woods Hole), Great Harbor (Woods Hole), Quissett Harbor and Megansett Harbor. Shellfish resource habitat maps highlight these water-bodies and are available at the Town of Falmouth Shellfish Department and the Conservation Commission office. The significant shellfish habitat areas are as follows: The Megansett Harbor area (1,049 acres) has significant populations of seeded quahogs, clams and oysters as well as occasional populations of bay scallops. West Falmouth Harbor (201 acres) is a significant resource for quahogs, soft-shell clams, oysters and scallops. The scallop population has been recently improved due to a local scallop program executed by the Shellfish Constable. Great Sippewissett Marsh (29 acres) has pockets of clams and quahogs, but has been experiencing degradation in water quality from runoff and septic systems, which has led to a prohibition on shellfishing. -

Waquoit Bay: Prehistory, History, and Natural History by Nancy Church
20 Waquoit Bay: Prehistory, History, and Natural History by Nancy Church Ellen Little and her brother Edward fshing from the beach on Washburn Island in the 1890s. Photo possibly by Edith Little. Courtesy D. W. Bourne. (Reprinted from Te Book of Falmouth.) Anniversaries are milestones and cause for celebration Research Reserve System. WBNERR uses the local and refection. 2013 marks the 25th Anniversary of estuary system as a living laboratory to conduct the creation of the Waquoit Bay National Estuarine and support research and to educate community Research Reserve (WBNERR), an organization members and decision makers across the region. Im- which manages almost 3,000 acres of land and water provements to the headquarters are underway now. in the towns of Mashpee and Falmouth. Work to Tis is an ideal time to revisit the long history of hu- establish the Reserve frst began in 1974 yet it wasn’t man activity around the Bay and the Reserve’s role in until 1988 that the deal was complete and Waquoit protecting coastal resources for future generations. Bay Reserve became part of the National Estuarine Te Reserve is a state and federal partnership between 20 21 the Massachusetts Department of Conservation and across the Cape with increased settlement over time, Recreation (DCR) and the National Oceanic and especially during the Woodland period 1500 to Atmospheric Administration (NOAA). It consists of 1,100 years ago when Native people lived in small the Headquarters and Visitor Center on the former villages or seasonal camps near abundant fresh and Sargent/Swift Estate, the 300 acre Washburn Island, salt water resources. -

The Release of Dissolved Actinium to the Ocean: a Global Comparison of Different End-Members
The release of dissolved actinium to the ocean: a global comparison of different end-members Walter Geibert, Matt Charette, Guebuem Kim, Willard S. Moore, Joseph Street, Megan Young, Adina Paytan Walter Geibert (corresponding author) previously Alfred Wegener Institute for Polar and Marine Research now at: School of GeoSciences, The University of Edinburgh, West Mains Road, Edinburgh EH9 3JW, UK, and the Scottish Association for Marine Science, Dunstaffnage Marine Laboratory, Dunbeg, Oban, PA37 1QA, UK Matt Charette Department of Marine Chemistry and Geochemistry Woods Hole Oceanographic Institution Woods Hole, MA 02543, USA Guebuem Kim School of Earth & Environmental Sciences Seoul National University Seoul 151-742, Korea Willard S. Moore Department of Geological Sciences University of South Carolina Columbia, SC 29208 Joseph Street Department of Geological and Environmental Sciences Green Earth Sciences, Room 056 Stanford, CA 94305-2115 Megan Young Isotope Tracers Project U.S. Geological Survey 345 Middlefield Road, MS 434 Menlo Park, CA 94025 USA Adina Paytan Department of Geological and Environmental Sciences Stanford University Stanford CA 94305-2115 Keywords: actinium, radium, submarine groundwater discharge, ocean mixing, continental shelf, global circulation models Abstract The measurement of short-lived 223 Ra often involves a second measurement for supported activities, which represents 227 Ac in the sample. Here we exploit this fact, presenting a set of 284 values on the oceanic distribution of 227 Ac, which was collected when analyzing water samples for short-lived radium isotopes by the radium delayed coincidence counting system. The present work compiles 227 Ac data from coastal regions all over the northern hemisphere, including values from ground water, from estuaries and lagoons, and from marine end- members. -

Town of Mashpee, Popponesset Bay, & Waquoit Bay East Watersheds
Town of Mashpee, Popponesset Bay, & Waquoit Bay East Watersheds TM Nitrex Technology Scenario Plan Submitted to: Town of Mashpee Submitted by: Sewer Commission 16 Great Neck Road North Mashpee, MA 02469 August 1, 2008 Town of Mashpee Sewer Commission August 1, 2008 Table of Contents Executive Summary........................................................................................................ 1 1 Objective................................................................................................................ 10 2 Background............................................................................................................ 10 2.1 Wastewater Flows .......................................................................................... 11 2.2 Hydrogeology ................................................................................................. 13 3 Needs Definition .................................................................................................... 20 3.1 Data Management - Methodology .................................................................. 20 3.2 Planning Area Parcel Summary...................................................................... 31 3.3 Nitrogen Loading and TMDLs......................................................................... 33 3.3.1 Data Overview......................................................................................... 34 3.3.2 Popponesset Bay Nitrogen Removal Requirements ............................... 38 3.3.3 East Waquoit Bay Nitrogen -

Popponesset Bay HIGH
WATERSHED REPORT: UPPER CAPE WATER THREAT LEVEL Popponesset Bay HIGH MASHPEE, BARNSTABLE & SANDWICH Introduction to the Watershed Reports In 2001, the Massachusetts Estuaries Project (MEP) was established to evaluate the health of 89 coastal embayment ecosystems across southeastern Massachusetts. A collaboration between coastal communities, the Massachusetts Department of Environmental Protection (MassDEP), the School of Marine Science and Technology (SMAST) at the University of Massachusetts-Dartmouth, the US Environmental Protection Agency (US EPA), the United States Geological Survey (USGS), the Massachusetts Executive Office of Energy and Environmental Affairs (EEA), and the Cape Cod Commission, the purpose of the MEP is to identify nitrogen thresholds and necessary nutrient reductions to support healthy ecosystems. The Cape Cod 208 Plan Update, certified and approved by the Governor of the Commonwealth of Massachusetts and the US EPA in 2015, provides an opportunity and a path forward to implement responsible plans for the restoration of the waters that define Cape Cod. On Cape Cod there are 53 embayment watersheds with physical characteristics that make them susceptible to nitrogen impacts. In its 2003 report, “The Massachusetts Popponesset Bay Watershed Estuaries Project – Embayment Restoration and Guidance for Implementation Strategies”, MassDEP identifies the 46 Cape Cod embayments included in the www.CapeCodCommission.org Implementation Report: Watershed Report October 2017 1 MEP. Thirty-three embayments studied to date require nitrogen reduction to could be evaluated. The Watershed Reports use the MEP recommendations for the achieve healthy ecosystem function. A Total Maximum Daily Load (TMDL) has required nitrogen load reductions necessary to meet the threshold loads (that serve been established (or a draft load has been identified and is under review) for these as the basis for nitrogen management), and then use the wMVP and the regionally watersheds. -

Applicant Town of Essex Project Director Alyssa B. Novak, Ph.D (Boston University; Provincetown Center for Coastal Studies)
CREATING THE BASIS FOR A SUCCESSFUL RESTORATION: TEST-TRANSPLANTING MULTIPLE EELGRASS DONOR POPULATIONS AND CHARACTERIZING THE EUROPEAN GREEN CRAB POPULATION TO INFORM RESTORATION INITIATIVES IN THE GREAT MARSH, MASSACHUSETTS Applicant Town of Essex Project Director Alyssa B. Novak, Ph.D (Boston University; Provincetown Center for Coastal Studies) Overview Eelgrass beds are an ecologically and economically valuable resource and have become the focus of management initiatives in Massachusetts. Great Marsh, which includes the water bodies of Plum Island Sound and Essex Bay, once contained acres of lush, thriving eelgrass beds that were wiped out by the mid-1900s. With funding from the Massachusetts Bays National Estuary Program (MassBays) in 2012 and 2013, our research team developed a model for Plum Island Sound that identified areas with good potential for the re-establishment and growth of eelgrass and we began test-transplanting eelgrass at the most suitable sites. In 2014, we expanded upon our prior work and began transplanting eelgrass in Essex Bay where a new self- established bed has been identified. In addition, we began collecting information on the population structure of the hyper-abundant European green crab population in Great Marsh in an effort to determine its potential impacts on our restoration initiatives. Eelgrass successfully transplanted into multiple sites in Essex Bay while transplants failed in Plum Island Sound. Green crab populations were found to be hyper-abundant throughout the Great Marsh system. Based on our results, we recommend conducting a large-scale restoration at select sites in Essex Bay using multiple donor sources while continuing test-transplanting efforts in Plum Island Sound. -

Chapter 1: Introduction
Waquoit Bay National Estuarine Research Reserve Management Plan 2014-2019 Supporting Coastal Communities through Science This Management Plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Massachusetts Coastal Zone Management, October 17, 2005. Acknowledgements This Management Plan was produced by current and former staff members of the Waquoit Bay National Estuarine Research Reserve: • Nancy Church, School and Interpretive Program Coordinator • Mary-Kay Fox, Former Assistant Research Coordinator • Alison Leschen, Former Reserve Manager • Jordan Mora, GIS/Research Technician • Joan Muller, Education Coordinator • Sheri Proft, Fiscal Administrative Assistant • James Rassman, Stewardship Coordinator\Acting Manager • John Singleton, Forest and Parks Supervisor • Tonna-Marie Surgeon-Rogers, Coastal Training Program Coordinator • Laurie Tompkins, Volunteer and Events Coordinator • Christopher Weidman, Research Coordinator The ad-hoc Reserve Advisory Committee met to help focus the Reserve’s priorities for the next five years. The committee included representatives from MA Department of Conservation and Recreation, MA Coastal Zone Management, Wood Hole Sea Grant, NOAA, Narragansett Bay NERR, NERRS Science Collaborative, Cape Cod Commission (the local Regional Planning Agency), Buzzards Bay National Estuary Program, the Mashpee Wampanoag Tribe, and several local environmental non-profits and community organizations. In addition, a Market Analysis/Needs Assessment for K-12 Education, and a CTP Needs Assessment were completed in 2011-12. Results of those studies have been incorporated into Goals and Objectives of this plan. Public Comment Period: July 1- 31, 2014. -
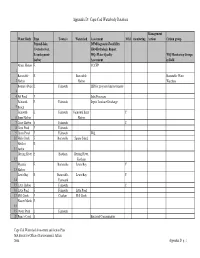
Appendix D: Cape Cod Waterbody Database
Appendix D: Cape Cod Waterbody Database Management Water Body Type Town(s) Watershed Assessment 303d monitoring Actions Citizen group F=pond-lake, D/F=Diagnostic-Feasibility C=creek-river, HR=Hydrologic Report E=embayment- WQ=Water Quality WQ Monitoring Groups harbor Assessment in Bold Allens Harbor E CCCEP 1 Barnstable E Barnstable Barnstable Water 2 Harbor Harbor Watchers Bourne's Pond E Falmouth EIR for proposed improvements 3 4 Eel Pond E Inlet Processes Falmouth E Falmouth Septic Leachate Discharge 5 Beach Falmouth E Falmouth Falmouth Inner Y 6 Inner Harbor Harbor 7 Great Harbor E Falmouth Y 8 Great Pond E Falmouth 9 Green Pond E Falmouth WQ, 10 Halls Creek E Barnstable Squaw Island Hatches E 11 harbor Herring River E Eastham Herring River, 12 Eastham Hyannis E Barnstable Lewis Bay Y 13 Harbor Lewis Bay E Barnstable- Lewis Bay Y 14 Yarmouth 15 Little Harbor E Falmouth Y 16 Little Pond E Falmouth Little Pond 17 Mill Creek E Chatham Mill Creek Nauset Marsh E 18 19 Oyster Pond E Falmouth 20 Paine's Creek E Bacterial Contamination Cape Cod Watershed Assessment and Action Plan MA Executive Office of Environmental Affairs 2004 Appendix D p. 1 Appendix D: Cape Cod Waterbody Database Management Water Body Type Town(s) Watershed Assessment 303d monitoring Actions Citizen group F=pond-lake, D/F=Diagnostic-Feasibility C=creek-river, HR=Hydrologic Report E=embayment- WQ=Water Quality WQ Monitoring Groups harbor Assessment in Bold Phinney's E 21 Harbor Pleasant Bay E Y Y Pleasant Bay Orleans Marine WQM Resource Task Force, Pleasant Bay Management -

Massachusetts Estuaries Project
MASSACHUSETTS ESTUARIES PROJECT Linked Watershed-Embayment Model to Determine Critical Nitrogen Loading Thresholds for the Oyster Pond System, Falmouth, Massachusetts University of Massachusetts Dartmouth Massachusetts Department of School of Marine Science and Technology Environmental Protection FINAL REPORT –JANUARY 2006 Massachusetts Estuaries Project Linked Watershed-Embayment Model to Determine Critical Nitrogen Loading Thresholds for Oyster Pond System, Falmouth, Massachusetts FINAL REPORT – January 2006 Brian Howes Roland Samimy David Schlezinger Sean Kelley John Ramsey Ed Eichner Contributors: US Geological Survey Don Walters and John Masterson Applied Coastal Research and Engineering, Inc. Elizabeth Hunt Massachusetts Department of Environmental Protection Brian Dudley (DEP project manager) SMAST Coastal Systems Program George Hampson, Sara Sampieri, Jen Antosca, and Michael Bartlett © [2006] University of Massachusetts All Rights Reserved ACKNOWLEDGMENTS The Massachusetts Estuaries Project Technical Team would like to acknowledge the contributions of the many individuals who have worked tirelessly for the restoration and protection of the critical coastal resources of the Oyster Pond System. Without these stewards and their efforts, this project would not have been possible. First and foremost is the significant time and effort in data collection and discussion spent by members of the Falmouth PondWatch Water Quality Monitoring Program, particularly B. Norris, J. Dowling, B. Livingstone, J. Rankin M. Zinn, D. Zinn. These individuals gave of their time to collect nutrient samples from this system over many years and without this information the present analysis would not have been possible. In addition, over the years the Oyster Pond Environmental Trust (OPET) has worked tirelessly with SMAST Coastal Systems Staff, engineers from Applied Coastal Research and Engineering and the Town of Falmouth Engineering Department in order to develop a management strategy for this system. -

Falmouth Navigable Waterways Dredging Areas a Pictorial View
Falmouth Navigable Waterways Dredging Areas A Pictorial View Draft 3 Jeff Thomas Falmouth Waterways Committee Dec 02, 2020 Falmouth Navigable Waterways Megansett Harbor Rands Canal Town of Falmouth Fiddlers Cove Massachusetts Wild Harbor • Has 95 miles of shoreline • Has 32 distinct West Falmouth Identified areas Harbor Child’s that are in the River Seapit past, currently, River or in the future Eel Waquoit Perch River Eel subject to Bournes Bay Pond West Pond River navigational Green East Great dredging Pond Pond Falmouth Inner Harbor Quissett Harbor Eel Pond Great Little Harbor Harbor 2 Falmouth Navigable Waterways Town of Falmouth Mass Dredgible Navigable Waterways Rev 4 12/02/2020 Moorings Location Area Qty Docks Clubs/Associations Boat Ramp Commercial Comments Megansett Approach Megansett Outer Hbr (MHO) 86 2 Megansett YC Town Ramp Sole access for Bourne Megansett Embayment Megansett Inner Hbr (MHI) 73 1 Town Megansett/Bourne Channel 8 Rands Canal Rands Canal (RC) 15 14 Fiddlers Approach Fiddlers Cove (FC) 5 32 Seascape Assoc Seascape Assoc Prvt Safe Habor Marina Fiddlers Embayment Wild Harbor Approach Wild Harbor Inner (WH) 37 1 Wild Hbr YC WHYC Prvt Wild Harbor Inlet Wild Harbor Outer (WHO) 60 Wild Harbor Embayment West Falmouth Approach West Fal Hbr (WF) 120 23 West Fal YC Town Ramp West Falmouth Inlet West Fal Outer (WFO) 197 Chappaquoit Assoc?? West Falmouth Embayment Quissett Embayment Quissett Harbor (QH) 248 20 Quissett YC Quissett Boat Yard Gansett Assoc Great Harbor Embayment Great Hbr Woods Hole (GHW) 218 30+ Woods Hole -

Waquoit Bay National Estuarine Research Reserve Management Plan 2006-2011
Waquoit Bay National Estuarine Research Reserve Management Plan 2006-2011 Supporting Coastal Communities through Science August, 2006 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Massachusetts Coastal Zone Management, October 17, 2005. Cover photograph: Looking southwest from above South Cape Beach at Sage Lot Pond, Waquoit Bay and Dead Neck. Acknowledgements This management plan was produced by Elizabeth Fuller Valentine, Management Plan Coordinator, and staff members of the Waquoit Bay National Estuarine Research Reserve: • Brendan Annett, Stewardship Coordinator and Acting Manager • MaryKay Fox, Assistant Research Coordinator • Christine Gault, Manager (former) • Pat Harcourt, Community Educator • Varia Harik, Fiscal Administrative Assistant • Joan Muller, Education Coordinator • Anne Reynolds, Technology Coordinator (former) • Joseph Souza, Forest and Parks Supervisor • John Singleton, Skilled Laborer • Tonna-Marie Surgeon-Rogers, Coastal Training Program Coordinator • Paul Ticco, Coastal Training Program Coordinator (former) • Laurie Tompkins, Events Coordinator • Christopher Weidman, Research Coordinator Citizens and public officials attended a public meeting in December 2004 to help focus the Reserve’s priorities for the next five years. Meeting participants included Rick York; Mashpee Shellfish Constable; -

Sediment Dynamics and Hydrographic Conditions During Storm Passage, Waquoit Bay, Massachusetts
Marine Geology 381 (2016) 67–86 Contents lists available at ScienceDirect Marine Geology journal homepage: www.elsevier.com/locate/margo Sediment dynamics and hydrographic conditions during storm passage, Waquoit Bay, Massachusetts Christopher V. Maio a,⁎, Jeffrey P. Donnelly b, Richard Sullivan b, Stephanie M. Madsen b, Christopher R. Weidman c, Allen M. Gontz d, Vitalii A. Sheremet e a University of Alaska Fairbanks, Department of Geosciences, PO Box 755780, Fairbanks, AK 99775, USA b Woods Hole Oceanographic Institution, Coastal Systems Group, 266 Woods Hole Road, Mail Stop #22, Woods Hole, MA 02543, USA c Waquoit Bay National Estuarine Research Reserve, 149 Waquoit Highway, Waquoit, MA 02536, USA d University of Massachusetts-Boston, School for the Environment, 100 Morrissey Blvd., Boston, MA 02125, USA e University of Rhode Island, Graduate School of Oceanography, 215 South Ferry Road, Narragansett, RI 02882, United States article info abstract Article history: The impact of storm events on the sediment dynamics of the shallow groundwater fed estuaries of Cape Cod, Mas- Received 16 November 2015 sachusetts, USA is little understood. To address this, the objectivesofthisstudyaretoassess sediment dynamics dur- Received in revised form 28 June 2016 ing storm passage and determine whether shallow back-barrier lagoons like Waquoit Bay have the preservation Accepted 29 July 2016 potential for a sedimentary archive of hurricanes. When setting out in this study, it was unclear whether Available online 31 July 2016 paleotempestological methods could be applied successfully to cores collected from the landward reaches of shallow estuaries of southern New England. Water level and bottom current data using Arm-and-Float tide gauges and Keywords: Sediment dynamics SeaHorse Tilt Current Meters was collected during Tropical Storm Irene (2011) and coupled with storm surge Storm surge modeling projections to better elucidate storm-induced sediment transport mechanisms.