Lithium Carbonate; [2] Lithium Chloride; [3] Lithium Hydroxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Clear-Chlor Lithium - 0040 Product Name: Clear-Chlor Lithium Date: 5/19/2015
SAFETY DATA SHEET Clear-Chlor Lithium - 0040 Product Name: Clear-Chlor Lithium Date: 5/19/2015 1. SECTION 1 IDENTIFICATION Supplier: Phoenix Products Company Distributor: 55 Container Drive Terryville, CT 06786 (860) 589-7502 U.S. PERS Emergency Telephone: 1-800-633-8253 Product Name: Clear-Chlor Lithium Synonyms: Hypochlorous acid, lithium salt; Lithium chlorideoxide; Lithium oxychloride; Hypure L Chemical Name: Lithium Hypochlorite Chemical Formula: ClLiO CAS Number: 13840-33-0 Product Use: Water sanitizer. 2. SECTION 2 HAZARDS IDENTIFICATION Emergency Overview Danger Corrosive Hazard Statement(s) H272: May intensify fire; oxidizer H314: Causes severe skin burns and eye damage H302: Harmful if swallowed H335: May cause respiratory irritation H410: Very toxic to aquatic life with long lasting effects Precautionary Statement(s) P210: Keep away from heat/sparks/open flames/hot surfaces. - No smoking P220: Keep/Store away from clothing/ combustible materials P221: Take any precaution to avoid mixing with combustibles/other chemicals P260: Do not breathe dust. P262: Do not get in eyes, on skin, or on clothing. P270: Do not eat, drink or smoke when using this product P271: Use only outdoors or in a well-ventilated area P273: Avoid release to the environment P280: Wear protective gloves/protective clothing/eye protection/face protection. P284: [In case of inadequate ventilation] wear respiratory protection. P233: Keep container tightly closed. P264: Wash thoroughly after handling. P363: Wash contaminated clothing before reuse P321: Specific treatment (see First Aid Measures on this label). P310: Immediately call a POISON CENTER or doctor/physician P391: Collect spillage P405: Store locked up P403+P233: Store in a well-ventilated place. -

Decontamination of Spas and Wading (Kiddie) Pools Revised – June 2011 Brought to You by the APSP Recreational Water Quality Committee
Fact Sheet Decontamination of Spas and Wading (Kiddie) Pools Revised – June 2011 Brought to you by the APSP Recreational Water Quality Committee I. INTRODUCTION The purpose of the fact sheet is to provide a generic overview of procedures to be used for the decontamination or annual maintenance of spas, hot tubs, swim spas and wading (kiddie) pools. If used for decontamination, the user/operator should check existing local or state regulatory agency guidelines. The following recommendations do not replace existing state or local guidelines. In the absence of regulatory agency guidelines, please refer to CDC Healthy Swimming recommendations. II. SUMMARY OF DECONTAMINATION CHARACTERISTICS 1. Decontaminate water, filter, plumbing 2. Drain, sanitize vessel, filter, plumbing then continue with water and /or filter media replacement and or treatment 3. Complete required documentation in cases involving an Accidental Fecal Release (AFR) accident or suspected bacterial outbreak or when decontamination is part of a general water replacement routine 4. Appropriate Personal Protective Equipment (PPE) should be worn by operators during the decontamination process. See Precautions section for more information. This APSP Fact Sheet has been prepared from the best information available at the time of its publication and represents a consensus of the members of the APSP Recreational Water Quality Committee. The APSP makes no guarantee, and assumes no liability, in connection with any of this information. Moreover, it should not be assumed that every acceptable procedure is included, or that special circumstances may not warrant modified or additional procedures. Appropriate steps should be taken to ensure that the information is current when used. -

Lithium Hypochlorite REGULAR USE DOSAGES: Corrosive
16.75 in SWIMMING POOL SANITIZER have settled to the bottom of the pool. center or doctor for treatment advice. IF IN EYES: Hold eye open and rinse BioGuard® has a wide variety of products that are required to maintain ALGAE TREATMENT TO REMOVE EXISTING ALGAE GROWTH: When slowly and gently with water for 15-20 minutes. Remove contact lenses, if a high quality swimming environment. These products are designated algae is seen, check and adjust, if needed, all water balance parameters. present, after the first 5 minutes, then continue rinsing eye. Call a poison as “Maintain.” Brush all surfaces with surface compatible brush. With circulation pump control center or doctor for treatment advice. IF INHALED: Move person Swimming pool water is subject to accumulation of a wide variety of operating, apply 2 lb of this product per 10,000 gallons of pool water by to fresh air. If person is not breathing, call 911 or an ambulance, then give organic contaminants: perspiration, suntan lotions, hair sprays and cos- broadcasting the product directly into the pool at deep end of pool. Use a artificial respiration, preferably mouth-to-mouth, if possible. Call poison metics. This quick-dissolving, non-clouding shock and superchlorinating pool brush to disperse granules which may have settled to the bottom of control center or doctor for treatment advice. product will effectively reduce organic contamination in swimming pool the pool to prevent damage to pool surfaces. Continue to operate recircu- Have the product container or label with you when calling a water and sanitize water, resulting in sparkling, clear water. -

Secretariat GENERAL
UNITED NATIONS ST Distr. Secretariat GENERAL ST/SG/AC.10/C.3/2002/33 12 April 2002 ORIGINAL : ENGLISH COMMITTEE OF EXPERTS ON THE TRANSPORT OF DANGEROUS GOODS AND ON THE GLOBALLY HARMONIZED SYSTEM OF CLASSIFICATION AND LABELLING OF CHEMICALS Sub-Committee of Experts on the Transport of Dangerous Goods (Twenty-first session, 1-10 July 2002 agenda item 4) TRANSPORT OF SOLID SUBSTANCES IN BULK IN CONTAINERS Transport of solids in portable tanks Transmitted by the expert from the United States of America 1. At the twentieth session of the Sub-Committee, the expert from the United States of America agreed to coordinate the development of requirements for the transport of solid dangerous goods in portable tanks and to lead a correspondence group to review proposed requirements and develop a proposal for submission to the twenty-first session. Many solids are not currently authorized for transport in portable tanks in the Model Regulations. Consignors that transport solid dangerous goods are required to acquire competent authority approvals to transport their solids in tanks which imposes unnecessary burdens and delays. Adoption of this proposal will result in the assignment of tank codes for the majority of solids dangerous goods listed in the Dangerous Goods List that are suitable and safe for transport in portable tanks. In developing the assignments, a rationalized approach was developed (see Annex 1) and other regulations that provide portable tank requirements for solids were considered (e.g. IMDG Code, ADR/RID and 49 CFR). Annex 2 of this paper provides proposed assignments that are based on the rationalized approach that is provided in Annex 1. -

Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: a Review
resources Review Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review Laurence Kavanagh * , Jerome Keohane, Guiomar Garcia Cabellos, Andrew Lloyd and John Cleary EnviroCORE, Department of Science and Health, Institute of Technology Carlow, Kilkenny, Road, Co., R93-V960 Carlow, Ireland; [email protected] (J.K.); [email protected] (G.G.C.); [email protected] (A.L.); [email protected] (J.C.) * Correspondence: [email protected] Received: 28 July 2018; Accepted: 11 September 2018; Published: 17 September 2018 Abstract: Lithium is a key component in green energy storage technologies and is rapidly becoming a metal of crucial importance to the European Union. The different industrial uses of lithium are discussed in this review along with a compilation of the locations of the main geological sources of lithium. An emphasis is placed on lithium’s use in lithium ion batteries and their use in the electric vehicle industry. The electric vehicle market is driving new demand for lithium resources. The expected scale-up in this sector will put pressure on current lithium supplies. The European Union has a burgeoning demand for lithium and is the second largest consumer of lithium resources. Currently, only 1–2% of worldwide lithium is produced in the European Union (Portugal). There are several lithium mineralisations scattered across Europe, the majority of which are currently undergoing mining feasibility studies. The increasing cost of lithium is driving a new global mining boom and should see many of Europe’s mineralisation’s becoming economic. The information given in this paper is a source of contextual information that can be used to support the European Union’s drive towards a low carbon economy and to develop the field of research. -

Sop Pyrophoric 2 12/16/2019
Owner DOC. NO. REV. DATE C.H.O SOP PYROPHORIC 2 12/16/2019 DOC. TITLE SOP FOR PYROPHORIC CHEMICALS Environmental Health & Safety STANDARD OPERATING PROCEDURES (SOP) FOR WORKING WITH PYROPHORIC CHEMICALS AT AMHERST COLLEGE ___________________________________________________________________ General Information Pyrophoric Chemicals are solid, liquid, or gas compounds that, when exposed to air or moisture at or below 54°C (130°F), can spontaneously ignite. Examples of Pyrophoric chemicals used at Amherst College Laboratories include: sodium hydride, zinc powder, and Grignard reagents. See the “Appendix” page below for a full list of Pyrophoric Chemicals. Pyrophoric chemicals are often used as catalysts in chemical reactions or as reducing and deprotonating agents in organic chemistry. Note that Pyrophoric chemicals may also be characterized by other hazards, hence, users of these chemicals may also need to refer to other SOPs that cover other hazards. In addition, each individual chemical’s Safety Data Sheet (SDS) should be consulted before they are used. _____________________________________________________________________________________ Personal Protective Equipment When working with Pyrophoric Chemicals, the following personal protective equipment (PPE) must be worn, at a minimum. Depending on the specific chemical, other forms of protection might be required. Consult the SDS for each chemical before use: Splash goggles Lab coat (Fire resistant lab coat highly recommended) Long pants Close toed shoes Gloves – Nitrile gloves adequate for accidental contact with small quantities. However, the use of fire resistant Nomex/ Leather Pilot’s gloves is highly recommended _____________________________________________________________________________________ Safety Devices All work with Pyrophoric chemicals must be done in a glove box, vacuum manifold, or any enclosed inert environment. If work must be done in a fume hood, ensure that the hood sash is in the lowest feasible position. -
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
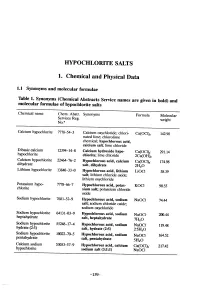
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

Extracorporeal Removal of Poisons and Toxins
CJASN ePress. Published on August 22, 2019 as doi: 10.2215/CJN.02560319 Extracorporeal Removal of Poisons and Toxins Joshua David King,1,2 Moritz H. Kern,3,4 and Bernard G. Jaar 5,6,7,8 Abstract Extracorporeal therapies have been used to remove toxins from the body for over 50 years and have a greater role than ever before in the treatment of poisonings. Improvements in technology have resulted in increased efficacy of removing drugs and other toxins with hemodialysis, and newer extracorporeal therapy modalities have expanded the role of extracorporeal supportive care of poisoned patients. However, despite these changes, for at least the 1Division of past three decades the most frequently dialyzed poisons remain salicylates, toxic alcohols, and lithium; in addition, Nephrology, the extracorporeal treatment of choice for therapeutic removal of nearly all poisonings remains intermittent University of hemodialysis. For the clinician, consideration of extracorporeal therapy in the treatment of a poisoning depends Maryland, Baltimore, Maryland; 2Maryland upon the characteristics of toxins amenable to extracorporeal removal (e.g., molecular mass, volume of Poison Center, distribution, protein binding), choice of extracorporeal treatment modality for a given poisoning, and when the Baltimore, Maryland; benefit of the procedure justifies additive risk. Given the relative rarity of poisonings treated with extracorporeal 3Department of therapies, the level of evidence for extracorporeal treatment of poisoning is not robust; however, extracorporeal Medicine, University Hospital Heidelberg, treatment of a number of individual toxins have been systematically reviewed within the current decade by the University of Extracorporeal Treatment in Poisoning workgroup, which has published treatment recommendations with an Heidelberg, improved evidence base. -

Heavy Metals & Essential Elements Testing in Dried
PROVIDER DATA SHEET Heavy Metals & Essential Elements Testing in Dried Urine & Dried Blood Spot We are all exposed to different amounts of essential and toxic elements depending on where we live, our diet and supplementation routine, or our lifestyle choices. Available Tests Levels of both essential and toxic elements that we consume or are exposed Toxic & Essential Elements – Urine to from the environment are determined by where we live, the water we drink, Tests included: Iodine, Selenium, Bromine, Lithium, Arsenic, Cadmium, Mercury, Creatinine the supplements we take, and the levels in soil/irrigation water used to grow the Assesses whether an individual has adequate, foods we eat. We are also exposed to toxic elements through environmental deficient, or excessive levels of the essential pollution of the air we breathe, as well as exposure through our skin. nutrients, or if they have been exposed to How do different levels of essential and toxic excessive levels of toxic heavy metals. elements affect health? Toxic & Essential Elements – Blood Essential elements are only conducive to optimal health when they are within Tests included: Cadmium, Mercury, Selenium, optimal ranges. Levels that are too low or too high can have detrimental effects Zinc, Magnesium, Copper, *Lead on health. Therefore, it is important to know if essential or toxic elements are *Research only outside their optimal ranges. Assesses whether an individual has adequate, deficient, or excessive levels of the essential Both iodine and selenium are good examples of essential elements that can be nutrients, or if they have been exposed to both beneficial and toxic, depending on their levels. -

Page 1 of 22
Page 1 of 22 Changes to the 2007 Right to Know Hazardous Substance List/Special Health Hazard Substance List Substance Common Name CAS DOT SHHC Sources Number Chemical Name … 0013 # ACETYL CHLORIDE 75-36-5 1717 CO F3 R2 3 8 15 17 20 21 ACETYL CHLORIDE … 0029 # AFLATOXINS 1402-68-2 CA MU TE 5 7 AFLATOXINS 3143 ALACHLOR 15972-60-8 2588 2 3 6 8 14 17 18 ACETAMIDE, 2-CHLORO-N-(2,6-DIETHYLPHENYL)-N-(METHOXYMETHYL)- … {syn ALLETHRIN see d-trans ALLETHRIN} 3647 d-trans-ALLETHRIN 28434-00-6 2902 3 6 17 18 {CYCLOPROPANECARBOXYLIC ACID, 2,2-DIMETHYL-3-(2-METHYL -1-PROPENYL)-, (1S)-2-METHYL-4-OXO-3-(2-PROPENYL)-2-CYCLOPENTEN-1-YL ESTER, (1R,3R)-} CYCLOPROPANECARBOXYLIC ACID, 2,2-DIMETHYL-3-(2-METHYL -1-PROPEN-1-YL)-, (1S)-2-METHYL-4-OXO-3-(2-PROPEN-1-YL)-2-CYCLOPENTEN-1-YL ESTER, (1R,3R)- … 0059 # ALUMINUM FLUORIDE 7784-18-1 1759 CO 1 2 3 4 17 ALUMINUM FLUORIDE (AlF3) … syn 1-(2-AMINOETHYL)PIPERAZIN see {AMINOETHYLPIPERAZINE} N-AMINOETHYLPIPERAZINE 0075 # {n-AMINOETHYLPIPERAZINE} N-AMINOETHYLPIPERAZINE 140-31-8 2815 CO 3 15 17 1-PIPERAZINEETHANAMINE Changes to the 2007 Right to Know Hazardous Substance List/Special Health Hazard Substance List are noted in RED. Deletions to the lists are contained within brackets { } and additions are underlined. Page 2 of 22 … 3154 # {2-(AMINO-5-(5-NITRO-2-FURYL))-1,3,4-THIADIAZOLE} 2-AMINO-5-(5-NITRO-2-FURYL)-1,3,4-THIADIAZOLE 712-68-5 CA 7 1,3,4-THIADIAZOL-2-AMINE, 5-(5-NITRO-2-FURANYL)- … 0083 # AMITROLE 61-82-5 2588 CA 2 3 4 5 6 7 8 14 17 18 20 21 1H-1,2,4-TRIAZOL-3-AMINE … 0086 # AMMONIUM ARSENATE 7784-44-3 -

Lithium Hypochlorite
United States Prevention, Pesticides EPA-738-F-93-018 Environmental Protection And Toxic Substances December 1993 Agency (7508W) R.E.D. FACTS Lithium Hypochlorite Pesticide All pesticides sold or distributed in the United States must be Reregistration registered by EPA, based on scientific studies showing that they can be used without posing unreasonable risks to people or the environment. Because of advances in scientific knowledge, the law requires that pesticides which were first registered years ago be reregistered to ensure that they meet today's more stringent standards. In evaluating pesticides for reregistration, EPA obtains and reviews a complete set of studies from pesticide producers, describing the human health and environmental effects of each pesticide. The Agency imposes any regulatory controls that are needed to effectively manage each pesticide's risks. EPA then reregisters pesticides that can be used without posing unreasonable risks to human health or the environment. When a pesticide is eligible for reregistration, EPA announces this and explains why in a Reregistration Eligibility Decision (RED) document. This fact sheet summarizes the information in the RED document for lithium hypochlorite. Use Profile Lithium hypochlorite is an algicide, disinfectant, fungicide and food contact surface sanitizer. Its primary pesticidal use is to control algae, bacteria and mildew in swimming pool water systems, hot tubs and spas; approximately 2,000,000 pounds of the active ingredient were used for this purpose in 1989. It also is used to sanitize food and cheese processing plant equipment, dairies, and eating establishment equipment and utensils. Lithium hypochlorite is formulated as a ready-to-use liquid and a soluble solid concentrate.