Bilateral Congenital Oral Mucous Extravasation Cysts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -

A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated Through 445 Nm Diode Laser
healthcare Case Report A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser Maria Vittoria Viani 1,*, Luigi Corcione 1, Chiara Di Blasio 2, Ronell Bologna-Molina 3 , Paolo Vescovi 1 and Marco Meleti 1 1 Department of Medicine and Surgery, University of Parma, 43126 Parma, Italy; [email protected] (L.C.); [email protected] (P.V.); [email protected] (M.M.) 2 Private practice, Centro Medico Di Blasio, 43121 Parma; Italy; [email protected] 3 Faculty of Dentistry, University of the Republic, 14600 Montevideo, Uruguay; [email protected] * Correspondence: [email protected] Received: 10 June 2020; Accepted: 11 August 2020; Published: 13 August 2020 Abstract: Oral granular cell tumor (GCT) is a relatively rare, benign lesion that can easily be misdiagnosed. Particularly, the presence of pseudoepitheliomatous hyperplasia might, in some cases, lead to the hypothesis of squamous cell carcinoma. Surgical excision is the treatment of choice. Recurrence has been reported in up to 15% of cases treated with conventional surgery. Here, we reported a case of GCT of the tongue in a young female patient, which was successfully treated through 445 nm diode laser excision. Laser surgery might reduce bleeding and postoperative pain and may be associated with more rapid healing. Particularly, the vaporization effect on remnant tissues could eliminate GCT cells on the surgical bed, thus hypothetically leading to a lower rate of recurrence. In the present case, complete healing occurred in 1 week, and no recurrence was observed after 6 months. Laser surgery also allows the possibility to obtain second intention healing. -
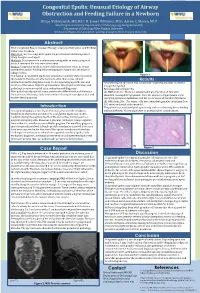
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H
Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Shilpa Vishwanath, MD,MS1; H. James Williams, MD2; Aaron C. Mason, MD3 1West Virginia University Department of Otolaryngology, Morgantown WV; 2Department of Pathology, West Virginia University 3Division of Plastic, Reconstructive, and Hand Surgery, West Virginia University Abstract Title: Congenital Epulis: Unusual Etiology of Airway Obstruction and Feeding Failure in a Newborn Objectives: Review congenital epulis; Its presentation and management. Study Design: Case Report Methods: Description of a newborn presenting with an obstructing oral mass. A review of the literature is included. Results: Congenital epulis is a rare oral lesion that may result in airway obstruction and/or feeding failure bringing the mass to the attention of subspecialists. Conclusion: A congenital epulis may present as a solitary alveolar mass in Figure 1 the newborn. Females are affected more often than males. Airway Results obstruction and feeding failure may evolve depending upon the size and The pathological specimen was a maxillary congenital granular cell tumor location of the lesion. Physical examination, radiographic evaluation, and (congenital epulis). pathologic review are useful in its evaluation and diagnosis. Pathology slides (Figure 3): Histopathologically, special stains assist in the differentiation of the lesion (A) H&E stain, 4x: There is a subepithelial proliferation of cells with from other solid tumors. Early intervention relieves airway obstruction and abundant eosinophilic cytoplasm. Note the absence of hyperplasia of the enables feeding success. overlying squamous epithelium and the prominence of vascular structures. (B) H&E stain, 20x: The tumor cells have abundant granular cytoplasm (low N/C ratio) and small uniform nuclei. -

Oral Mucocele – Diagnosis and Management
Journal of Dentistry, Medicine and Medical Sciences Vol. 2(2) pp. 26-30, November 2012 Available online http://www.interesjournals.org/JDMMS Copyright ©2012 International Research Journals Review Oral Mucocele – Diagnosis and Management Prasanna Kumar Rao 1, Divya Hegde 2, Shishir Ram Shetty 3, Laxmikanth Chatra 4 and Prashanth Shenai 5 1Associate Professor, Department of Oral Medicine and Radiology, Yenepoya Dental College, Yenepoya University, Deralakatte, Nithyanandanagar Post, Mangalore, Karnataka, India. 2Assistant Professor, Department of Obstetrics and Gynecology, AJ Institute of Medical Sciences, Mangalore, Karnataka, India. 3Reader, Department of Oral Medicine and Radiology, AB Shetty Memorial Institute of Dental Sciences, Nitte University, Mangalore, Karnataka, India. 4Senior Professor and Head, Department of Oral Medicine and Radiology, Yenepoya Dental College, Yenepoya University, Deralakatte, Nithyanandanagar Post, Mangalore, Karnataka, India. 5Senior Professor, Department of Oral Medicine and Radiology, Yenepoya Dental College, Yenepoya University, Deralakatte, Nithyanandanagar Post, Mangalore, Karnataka, India. ABSTRACT Mucocele are common salivary gland disorder which can be present in the oral cavity, appendix, gall bladder, paranasal sinuses or lacrimal sac. Common location for these lesions in oral cavity is lower lip however it also presents on other locations like tongue, buccal mucosa, soft palate, retromolar pad and lower labial mucosa. Trauma and lip biting habits are the main cause for these types of lesions. These are painless lesions which can be diagnosed clinically. In this review, a method used for searching data includes various internet sources and relevant electronic journals from the Pub Med and Medline. Keywords: Mucocels, Lower lip, Retention cyst. INTRODUCTION Mucocele is defined as a mucus filled cyst that can Types appear in the oral cavity, appendix, gall bladder, paranasal sinuses or lacrimal sac (Baurmash, 2003; Clinically there are two types, extravasation and retention Ozturk et al., 2005). -

Case Report Gingival Cyst of the Adult As Early Sequela of Connective Tissue Grafting
Hindawi Publishing Corporation Case Reports in Dentistry Volume 2015, Article ID 473689, 6 pages http://dx.doi.org/10.1155/2015/473689 Case Report Gingival Cyst of the Adult as Early Sequela of Connective Tissue Grafting Mariana Gil Escalante1,2 and Dimitris N. Tatakis1 1 Division of Periodontology, College of Dentistry, The Ohio State University, Columbus, OH 43210, USA 2Private Practice, San Jose, Costa Rica Correspondence should be addressed to Dimitris N. Tatakis; [email protected] Received 19 May 2015; Accepted 23 June 2015 Academic Editor: Jiiang H. Jeng Copyright © 2015 M. Gil Escalante and D. N. Tatakis. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The subepithelial connective tissue graft (SCTG) is a highly predictable procedure with low complication rate. The reported early complications consist of typical postsurgical sequelae, such as pain and swelling. This case report describes the development and management of a gingival cyst following SCTG to obtain root coverage. Three weeks after SCTG procedure, a slightly raised, indurated, ∼5 mm diameter asymptomatic lesion was evident. Excisional biopsy was performed and the histopathological evaluation confirmed the gingival cyst diagnosis. At the 1-yearollow-up, f the site had complete root coverage and normal tissue appearance and the patient remained asymptomatic. 1. Introduction This report presents a hitherto unreported early com- plication of SCTG, namely the development of a gingival The subepithelial connective tissue graft (SCTG) procedure, cyst of the adult (GCA), describes the management of this first introduced for root coverage in 19851 [ , 2], is considered complication and reviews similar postoperative sequelae. -

BIMJ April 2013
Original Article Brunei Int Med J. 2013; 9 (5): 290-301 Yellow lesions of the oral cavity: diagnostic appraisal and management strategies Faraz MOHAMMED 1, Arishiya THAPASUM 2, Shamaz MOHAMED 3, Halima SHAMAZ 4, Ramesh KUMARASAN 5 1 Department of Oral & Maxillofacial Pathology, Dr Syamala Reddy Dental College Hospital & Research Centre, Bangalore, India 2 Department of Oral Medicine & Radiology, Dr Syamala Reddy Dental College Hospital & Research Centre, Bangalore, India 3 Department of Community & Public Health Dentistry, Faculty of Dentistry, Amrita University, Cochin, India 4 Amrita center of Nanosciences, Amrita University, Cochin, India 5 Oral and Maxillofacial Surgery, Faculty of Dentistry, AIMST University, Kedah, Malaysia ABSTRACT Yellow lesions of the oral cavity constitute a rather common group of lesions that are encountered during routine clinical dental practice. The process of clinical diagnosis and treatment planning is of great concern to the patient as it determines the nature of future follow up care. There is a strong need for a rational and functional classification which will enable better understanding of the basic disease process, as well as in formulating a differential diagnosis. Clinical diagnostic skills and good judgment forms the key to successful management of yellow lesions of the oral cavity. Keywords: Yellow lesions, oral cavity, diagnosis, management INTRODUCTION INTRODUCTI Changes in colour have been traditionally low lesions have a varied prognostic spec- used to register and classify mucosal and soft trum. The yellowish colouration may be tissue pathology of the oral cavity. Thus, the- caused by lipofuscin (the pigment of fat). It se lesions have been categorised as white, may also be the result of other causes such red, white and red, blue and/or purple, as accumulation of pus, aggregation of lym- brown, grey and/or black and yellow. -

Gingival Cyst of Adults- Two Case Reports and Literature Review
https://doi.org/10.5272/jimab.2018242.2065 Journal of IMAB Journal of IMAB - Annual Proceeding (Scientific Papers). 2018 Apr-Jun;24(2) ISSN: 1312-773X https://www.journal-imab-bg.org Case reports GINGIVAL CYST OF ADULTS- TWO CASE REPORTS AND LITERATURE REVIEW Elitsa Deliverska1, Aleksandar Stamatoski2 1) Department of Oral and Maxillofacial Surgery, Faculty of Dental Medicine, Medical University – Sofia, Bulgaria. 2) Department of maxillofacial surgery, Faculty of Dental Medicine, Ss. Cyril and Methodius University- Skopje, Macedonia. ABSTRACT usually found in the incisor, canine, and premolar areas. Background: Gingival cyst of adult is an [1, 3, 4] uncommon, small, non inflammatory, extra-osseous, Clinically, the gingival cysts may certainly occur developmental cyst of gingiva arising from the rests of without bone involvement and may appear as painless, dental lamina. small sessile soft tissue swellings, usually involving the Purpose: The aim of our paper is to present two rare interdental area of the attached gingiva. clinical cases of gingival cyst of adult. These lesions measure about 0.5 to 1 cm in diameter. Material and methods: In the present cases, the They are often bluish or blue-gray due to thinning of the combined anatomic characteristics of the soft tissue overlying mucosa. In some instances, the cyst may cause presentation and the osseous defect suggest that the lesion slight erosion of the surface of the bone, which is usually is a gingival cyst of adult. Two cases of gingival cyst were not detected on a radiograph but is apparent during surgical diagnosed and treated with exicisional biopsy followed by exploration. -

Treatments for Ankyloglossia and Ankyloglossia with Concomitant Lip-Tie Comparative Effectiveness Review Number 149
Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Comparative Effectiveness Review Number 149 Treatments for Ankyloglossia and Ankyloglossia With Concomitant Lip-Tie Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. 290-2012-00009-I Prepared by: Vanderbilt Evidence-based Practice Center Nashville, TN Investigators: David O. Francis, M.D., M.S. Sivakumar Chinnadurai, M.D., M.P.H. Anna Morad, M.D. Richard A. Epstein, Ph.D., M.P.H. Sahar Kohanim, M.D. Shanthi Krishnaswami, M.B.B.S., M.P.H. Nila A. Sathe, M.A., M.L.I.S. Melissa L. McPheeters, Ph.D., M.P.H. AHRQ Publication No. 15-EHC011-EF May 2015 This report is based on research conducted by the Vanderbilt Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-2012-00009-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. -

Pigmented Villonodular Synovitis of the Temporomandibular Joint: a Case Report and the Literature Review
1314 Cai et al. Case Report TMJ Disorders J. Cai1, Z. Cai1, Y. Gao2 1Department of Oral and Maxillofacial Pigmented villonodular synovitis 2 Surgery, Beijing, China; Department of Oral Pathology, Peking University School & of the temporomandibular joint: Hospital of Stomatology, Beijing, China a case report and the literature review J. Cai, Z. Cai, Y. Gao: Pigmented villonodular synovitis of the temporomandibular joint: a case report and the literature review. Int. J. Oral Maxillofac. Surg. 2011; 40: 1314–1322. # 2011 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved. Abstract. Pigmented villonodular synovitis (PVNS) is an uncommon benign proliferative disorder of synovium that may involve joints, tendon sheaths, and bursae. It most often affects the knees, and less frequently involves other joints. It presents in the temporomandibular joints (TMJs) extremely rarely. The authors report an elderly female patient with PVNS of the TMJ with skull base extension, who had traumatic history in the same site. It was diagnosed through core-needle Keywords: pigmented villonodular synovitis (PVNS); synovitis; temporomandibular joint biopsy, which was not documented in the literature. Radical excision and follow-up (TMJ). for 7–8 years was recommended because of the reported malignant transformation and high recurrence rate. This case and previously reported cases in the literature are Accepted for publication 2 March 2011 reviewed and discussed. Available online 6 April 2011 The term pigmented villonodular synovi- were reported in detail (Table 1). The visits and mouth-opening for a long time tis (PVNS) was introduced by JAFFE et al. authors present an additional case of during the operation. -

Oral Pathology Final Exam Review Table Tuanh Le & Enoch Ng, DDS
Oral Pathology Final Exam Review Table TuAnh Le & Enoch Ng, DDS 2014 Bump under tongue: cementoblastoma (50% 1st molar) Ranula (remove lesion and feeding gland) dermoid cyst (neoplasm from 3 germ layers) (surgical removal) cystic teratoma, cyst of blandin nuhn (surgical removal down to muscle, recurrence likely) Multilocular radiolucency: mucoepidermoid carcinoma cherubism ameloblastoma Bump anterior of palate: KOT minor salivary gland tumor odontogenic myxoma nasopalatine duct cyst (surgical removal, rare recurrence) torus palatinus Mixed radiolucencies: 4 P’s (excise for biopsy; curette vigorously!) calcifying odontogenic (Gorlin) cyst o Pyogenic granuloma (vascular; granulation tissue) periapical cemento-osseous dysplasia (nothing) o Peripheral giant cell granuloma (purple-blue lesions) florid cemento-osseous dysplasia (nothing) o Peripheral ossifying fibroma (bone, cartilage/ ossifying material) focal cemento-osseous dysplasia (biopsy then do nothing) o Peripheral fibroma (fibrous ct) Kertocystic Odontogenic Tumor (KOT): unique histology of cyst lining! (see histo notes below); 3 important things: (1) high Multiple bumps on skin: recurrence rate (2) highly aggressive (3) related to Gorlin syndrome Nevoid basal cell carcinoma (Gorlin syndrome) Hyperparathyroidism: excess PTH found via lab test Neurofibromatosis (see notes below) (refer to derm MD, tell family members) mucoepidermoid carcinoma (mixture of mucus-producing and squamous epidermoid cells; most common minor salivary Nevus gland tumor) (get it out!) -

Management of Gingival Cyst of a Newborn: a Case Report with Epidemiological Data. Fathimath Fairooz1 †, Yaafiya Saleem2†, Md
Case report Management of gingival cyst of a newborn: a case report with epidemiological data. Fathimath Fairooz1 †, Yaafiya saleem2†, Md. Ashif Iqbal3* ABSTRACT: AFFILIATION: Introduction: Gingival cyst also known as Dental Lamina cyst. They form small 1. Fathimath Fairooz† nodules or cysts on the alveolar ridge, each up to 2 mm in diameter. They are Graduated from Update Dental College, Bangladesh considered to be due to proliferation of the epithelial rests of Serres but can BM&DC No: 12543 arise on the lateral aspects of the ridge and the crest. They are considered to Address: Fehivillage, HA, Dhidhoo, Maldives be benign lesions which usually are present on the alveolar ridge and are often Mail: [email protected] mistaken as neonatal tooth.Objective: The aim of this case report is to enlist Contact no: +9609722989 and describe the diagnostic features and management of the gingival cyst of https://orcid.org/0000-0001-7637-9705 newborn, as these kind of lesions can cause anxiety and fear among the parents. It is important to diagnose these conditions in order to prevent † 2. Yaafiya Saleem unnecessary surgical interventions and provide adequate care and proper Graduated from Update Dental College, Bangladesh knowledge to the parents. Method: This is a case study of asymptomatic BM&DC No: 12532 gingival cyst which was present in a newborn at the time of birth on the lower Address: Veenus aage, G.Dh fiyoaree, Maldives alveolar ridge. Diagnosis of this keratin filled small cystic lesion was made on Mail: [email protected] the basis of clinical history and oral findings.