For Review Only Page 3 of 22
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Open Research Online Oro.Open.Ac.Uk
Open Research Online The Open University’s repository of research publications and other research outputs Development and validation of the relational depth frequency scale Journal Item How to cite: Di Malta, Gina; Evans, Chris and Cooper, Mick (2019). Development and validation of the relational depth frequency scale. Psychotherapy Research, 30(2) pp. 213–227. For guidance on citations see FAQs. c 2019 Society for Psychotherapy Research https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Accepted Manuscript Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.1080/10503307.2019.1585590 Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Development and Validation of The Relational Depth Frequency Scale Gina Di Malta University of Roehampton Chris Evans University of Sheffield Mick Cooper University of Roehampton Gina Di Malta Department of Psychology, University of Roehampton, Holybourne Avenue, London SW15 4JD, 07871683006, [email protected] Chris Evans Department of Psychology, University of Sheffield, Western Bank, Sheffield S10 2TN, [email protected] Mick Cooper Department of Psychology, University of Roehampton, Holybourne Avenue, London SW15 4JD, 07734-558155, [email protected] Reference Di Malta, G., Evans, C., & Cooper, M. (2019). Development and validation of the relational depth frequency scale. Psychotherapy Research. DOI: 10.1080/10503307.2019.1585590 Acknowledgements Thanks to Rosanne Knox, David Murphy, Sue Price, Shiri Gurvitz, Ariele Noble, Eddie Chauncy, Aisling Treanor Donnelly, Joel Vos, and Regina Pauli for their contributions to the development of the Relational Depth Frequency Scale. -

Choosing a University/Course 50,000 There Are Around 50,000 130 40 Undergraduate Courses to Universities in the UK Universities in London Choose from in the UK
Choosing a University/Course 50,000 There are around 50,000 130 40 undergraduate courses to Universities in the UK Universities in London choose from in the UK. You can apply for up to 5 courses however you only write 1 personal statement. That is why it is important to find courses in a similar area so that your personal statement sounds concise and demonstrates your interest in that particular subject. Choosing a Course – 4 things to think about University is focused on teaching and learning. Going to university is an opportunity to immerse yourself in a subject you are genuinely fascinated by, want to find out more about, or that you want a career in. There are a few things to consider when picking what course you’d like to study. 1. Favourite Subject 3. Career Ambitions What topics do you enjoy studying? Can you What job do you want in the future? What study that topic at University? Would you like industry would you like to work in? Is earning to study that subject full time? Will the subject a good salary important to you? If you know matter be similar to your current studies? Are you want to be a doctor, a nurse, an architect you good at that subject? or a vet then there are set degree programmes you will need to study. However, for the most part, employers aren’t interested in what 2. Personal Interests degree programme you studied, they are more interested in the huge array of skills that a What do you like to do in your spare time or as a graduate brings to the work place. -

Annual Report and Financial Statements 2019/20
Annual Report and Financial Statements 2019/20 Roehampton University Company Registration Number 5161359 (England and Wales) Contents Chair of Council’s Welcome ..................................................................................... 4 Strategic Report ........................................................................................................... 6 Key Performance Indicators.................................................................................. 8 Financial Review ....................................................................................................10 Student Experience ...............................................................................................12 Staff Experience.....................................................................................................15 Learning, Teaching and Student Success ........................................................16 Research .................................................................................................................18 Outreach, Participation and Community Engagement .................................. 20 Responsible University .........................................................................................22 Risk and Uncertainty .............................................................................................24 Members of Council Report ...................................................................................26 Statement of Public Benefit.................................................................................28 -

Finding the Right Management Approach in Independent Hotels
Middlesex University Research Repository An open access repository of Middlesex University research http://eprints.mdx.ac.uk Nazarian, Alireza, Atkinson, Peter, Foroudi, Pantea ORCID: https://orcid.org/0000-0003-4000-7023 and Dennis, Karen (2019) Finding the right management approach in independent hotels. International Journal of Contemporary Hospitality Management, 31 (7) . pp. 2862-2883. ISSN 0959-6119 [Article] (doi:10.1108/IJCHM-08-2018-0700) Final accepted version (with author’s formatting) This version is available at: https://eprints.mdx.ac.uk/26177/ Copyright: Middlesex University Research Repository makes the University’s research available electronically. Copyright and moral rights to this work are retained by the author and/or other copyright owners unless otherwise stated. The work is supplied on the understanding that any use for commercial gain is strictly forbidden. A copy may be downloaded for personal, non-commercial, research or study without prior permission and without charge. Works, including theses and research projects, may not be reproduced in any format or medium, or extensive quotations taken from them, or their content changed in any way, without first obtaining permission in writing from the copyright holder(s). They may not be sold or exploited commercially in any format or medium without the prior written permission of the copyright holder(s). Full bibliographic details must be given when referring to, or quoting from full items including the author’s name, the title of the work, publication details where relevant (place, publisher, date), pag- ination, and for theses or dissertations the awarding institution, the degree type awarded, and the date of the award. -

Doctor of Education (Edd)
UNIVERSITY OF ROEHAMPTON & KINGSTON UNIVERSITY Doctor of Education (EdD) (2011 regulations) 1 Table of Contents Page Rationale 4 The doctoral status of the award 4 The providers 6 Programme aims 6 Learning Outcomes 6 Learning and Teaching 7 Registration on the programme 10 Assessment 12 Assessment criteria and marking scale 17 Preparation and Presentation of Research Proposal module 18 Research Project module 19 The management of the programme 21 Library and learning resources 23 Ethics 25 Complaints and Grievance procedure 26 Equality 26 Student support and guidance 28 Fees 29 Quality Assurance 29 Curriculum map 30 2 The Intermediate award: Masters in Education 31 The research environment in the partner universities 32 Programme team contact details 34 Select bibliography 35 Module framework diagram 37 Research Skills Audit: Self-Assessment 38 End of year action planning Years 1 and 2 40 NB: Every attempt has been made to ensure that the information contained in this handbook is accurate. We believe it to be correct at the time of printing. However, changing circumstances may require minor changes (as, for example, to membership of teaching teams), throughout the year. If you find the handbook to be misleading in any particular, or if you identify an error or inaccuracy, please bring this to the attention of the programme directors (Dr Julie Shaughnessy; Dr Keith Grieves) so that the necessary action can be taken. 3 DOCTOR OF EDUCATION (EdD) Welcome to the programme Rationale The Doctor of Education is a joint programme offered by Kingston University and the University of Roehampton. It is professionally focused and combines contextual modules with independent research and leads to a doctoral degree. -

College and University Acceptances
COLLEGE AND UNIVERSITY ACCEPTANCES 2016 -2020 UNITED KINGDOM • University of Bristol • High Point University • Aberystwyth University • University of Cardiff • Hofstra University • Arts University Bournemouth • University of Central Lancashire • Ithaca College • Aston University • University of East Anglia • Lesley University • Bath Spa University • University of East London • Long Island University - CW Post • Birkbeck University of London • University of Dundee • Loyola Marymount University • Bornemouth University • University of Edinburgh • Loyola University Chicago • Bristol, UWE • University of Essex • Loyola University Maryland • Brunel University London • University of Exeter • Lynn University • Cardiff University • University of Glasgow • Marist College • City and Guilds of London Art School • University of Gloucestershire • McGill University • City University of London • University of Greenwich • Miami University • Durham University • University of Kent • Michigan State University • Goldsmiths, University of London • University of Lancaster • Missouri Western State University • Greenwich School of Management • University of Leeds • New York Institute of Technology • Keele University • University of Lincoln • New York University • King’s College London • University of Liverpool • Northeastern University • Imperial College London • University of Loughborough • Oxford College of Emory University • Imperial College London - Faculty of Medicine • University of Manchester • Pennsylvania State University • International School for Screen -
Where Are They Now? from Ashmole
WHERE ARE THEY NOW? FROM ASHMOLE BRITISH AND IRISH MODERN MUSIC INSTITUTE ACADEMY COVENTRY UNIVERSITY TO BARNET AND KEELE UNIVERSITY KINGSTON UNIVERSITY LONDON SOUTHGATE LONDON SOUTH BANK UNIVERSITY COLLEGE TO... MIDDLESEX UNIVERSITY QUEEN MARY UNIVERSITY OF LONDON ROYAL HOLLOWAY UNIVERSITY OF BRIGHTON UNIVERSITY OF EAST LONDON UNIVERSITY OF HERTFORDSHIRE UNIVERSITY OF HULL UNIVERSITY OF KENT UNIVERSITY OF PORTSMOUTH UNIVERSITY OF ROEHAMPTON UNIVERSITY OF SURREY UNIVERSITY OF THE ARTS LONDON UNIVERSITY OF WESTMINSTER 020 8266 4000 [email protected] www.barnetsouthgate.ac.uk WHERE ARE THEY NOW? FROM ASHMOLE ACADEMY TO BARNET AND SOUTHGATE COLLEGE TO? NILDA ANTHONY GEORGE REBECCA MUSLU CHEN COONEY BUTWRIGHT ACCOUNTING & FINANCE HISTORY COMPUTER SCIENCE PHOTOGRAPHY MIDDLESEX UNIVERSITY UNIVERSITY OF PORTSMOUTH UNIVERSITY OF SURREY UNIVERSITY OF BRIGHTON NATASHA ZAHID PRAKAASH MANGALPARA NIZAMI BUSINESS MANAGEMENT ELECTRONIC ENGINEERING KAMALANATHAN KINGSTON UNIVERSITY UNIVERSITY OF INTERNATIONAL TOURISM MANAGEMENT LONDON WESTMINSTER MIDDLESEX UNIVERSITY MUHSIN BEN SAHDHATH YESILADA PRESCOTT PSYCHOLOGY BUSINESS MANAGEMENT ROYAL HOLLOWAY AND ENGLISH GAFOOR KEELE UNIVERSITY MULTIMEDIA BROADCAST JOURNALISM KIERAN RIO RAWL CHINWADA- UNIVERSITY OF BRIGHTON COMPUTER SCIENCE ONANUGA UNIVERSITY OF KENT NURSING (ADULT) MIDDLESEX UNIVERSITY IAN AMAR HARRY COOPER HUSSEIN DIAGNOSTIC RADIOGRAPHY GRAPHIC DESIGN SCORDELLIS AND IMAGING MIDDLESEX UNIVERSITY PHYSICS WITH FOUNDATION UNIVERSITY OF HERTFORDSHIRE UNIVERSITY OF HULL WHERE ARE THEY NOW? -

Thesis Presentation Guidance
THESIS PRESENTATION GUIDANCE Once you been entered for the examination you will be required to submit three copies of your thesis (unless advised otherwise), along with an RDB10 candidates declaration form to the Graduate School Office within your maximum period of registration. You can submit this in person or by secure mail. If your thesis is being delivered to the University by a third party please use the following details: Graduate School Grove House 104 Froebel College University of Roehampton Roehampton Lane SW15 5PH The three copies of your thesis should be temporarily bound (eg. spiral, comb or thermal bound). Subsidiary papers and other material forming part of the thesis (including tapes or videos) should be bound into the final thesis if it is possible or securely attached to your thesis and clearly labelled with your name. A list of suggested thesis binding companies is available on the Graduate School Moodle site. The text should be written in English (unless the University's Research Degrees Board has given permission for another language to be used owing to the nature of the subject). Other regulatory requirements on the presentation of theses are listed below: Paper should be A4, white and within the range of 70g/m2 to 100g/m2. There should be a margin of 1½ inches (4 cm) on the left hand side of the pages to allow for the binding. Other margins should not be less than 15mm. Running heads and page numbers should be within the recommended margins. The height of the characters used in the main text, including displayed matter and notes, should not be less than 2.0mm for capital letters and 1.5mm for a lower case x. -
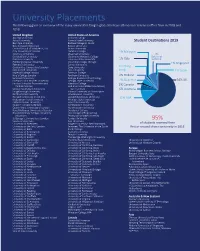
University Placements the Following Gives an Overview of the Many Universities Tanglin Graduates Have Attended Or Received Offers from in 2018 and 2019
University Placements The following gives an overview of the many universities Tanglin graduates have attended or received offers from in 2018 and 2019. United Kingdom United States of America Abertay University Amherst College Aston University Arizona State University Student Destinations 2019 Bath Spa University Berklee College of Music Bournemouth University Boston University Central School of St Martin’s, UAL Brown University City, University of London Carleton College 1% Malaysia Coventry University Chapman University 7% De Montfort University Claremont McKenna College National Durham University Colorado State University 1% Italy Service Edinburgh Napier University Columbia College, Chicago Falmouth University Cornell University 1% Singapore Goldsmiths, University of London Duke University 1% Hong Heriot-Watt University Elon University Kong 1% Spain Imperial College London Emerson College King’s College London Fordham University 2% Holland Lancaster University Georgia Institute of Technology Liverpool John Moores University Georgia State University 4% Gap Year 64% UK London School of Economics and Hamilton College Political Science Hult International Business School, 2% Canada London South Bank University San Francisco 6% Australia Loughborough University Indiana University at Bloomington Northumbria University John Hopkins University Norwich University of the Arts Loyola Marymount University 11% USA Nottingham Trent University Michigan State University Oxford Brookes University New York University Queen’s University Belfast Northeastern -

Mdx-Seminar 3.Pptx
L III A Communicave Interacon / N Literacy and Exper+se G M SIC OF LANGUAGE U A G E 2010 Malobe O F Women Singing M U Mbendjele S © J. Lewis: I C London 5-6 Sept 2011 Organised by Middlesex Language & Communicaon Research Group & Cambridge Centre for Music and Science seminar 3: communicative interaction/ literacy & expertise Introduc)on to the seminar series This seminar series aims to explore new direc3ons in the study of the relaonship between language and music in psychology. Language and music share many features: they are both composed of sequences of sub-units; both require these units to be organised according to conven3onal structural rules; they vary pitch, temporal and rhythmic features to convey meaning; they u3lize specific wri3ng systems; and they both organize coordinated or joint social ac3ons. The debate is s3ll open concerning the evolu3onary relaonship between language and music, their boundaries (what is unique and specific to each) and whether they do or do not belong to separate domains/modules. For instance, Brown (2000) proposed that language and music may be described as two end-points of a con3nuum in which sound would specialize to convey more affec3ve meaning (music) or referen3al meanings (language). This suggests that language and music are evolu3onary homologues stemming from a musilanguage common ancestor (see Cross, 2005; 2007; Fitch, 2005, 2006; Mithen, 2006 aer Darwin 1871). These ideas have s3mulated hypotheses in areas ranging from the neurological bases of language and music, to transfers of learning across the two domains, the use of music in neurological rehabilitaon and other clinical contexts, as well as theore3cal issues of embodied cogni3on and joint ac3on. -

Quality Assurance and Enhancement Committee (QAEC) Minutes of The
Quality Assurance and Enhancement Committee (QAEC) Minutes of the meeting held on Tuesday 10th November 2015 Present Professor Debra Humphris, Vice Provost (Education) - Chair Professor Peter Cheung, Vice Dean (Education), Faculty of Engineering Professor Sue Gibson, Director of the Graduate School Professor Tony Magee, Deputy Director of the Graduate School Dr Edgar Meyer, Associate Dean of Undergraduate Programmes, Business School Mr Chun-Yin San, Imperial College Union, Deputy President (Education) Professor Sue Smith, Chair of the Medical Studies Committee, Faculty of Medicine Professor Denis Wright, Director of Student Support Ms Sophie White, Senior Assistant Registrar (Quality Assurance & Enhancement) - Secretary In attendance: Ms Liz Chang, University of Roehampton (visitor) Mr Chris Harris, Quality and Educational Development Manager, Faculty of Medicine Ms Sarah Henderson, Helix Manager (for item 6.1) Dr Matthew Harris, Senior Policy Fellow in Public Health, IGHI, (for item 6.1) Apologies: Mr Liucheng Guo, Graduate Students’ Union President Professor Myra McClure, Senior College Consul Mr Dean Pateman, Academic Registrar Professor Alan Spivey, Vice Dean (Education), Faculty of Natural Sciences 1. Welcome and Apologies The Chair welcomed attendees to the meeting. It was noted that Dr Edgar Meyer, Associate Dean for Undergraduate Programmes was replacing Ms Diane Morgan, Professor Anthony Magee, Deputy Chair of the Graduate School was replacing Dr David McPhail and Professor Myra McClure, Senior College Consul was replacing Professor Richard Thompson. The committee also welcomed Ms Liz Chang from the University of Roehampton who was work shadowing the Secretary. Apologies as listed above were noted. 2. Minutes of the Previous Meeting QAEC.2015.01 The unconfirmed minutes from the Quality Assurance & Enhancement QAEC.2015.01a Committee (QAEC) held on Tuesday 14th July 2015 were approved. -

University Acceptances WAB Class of 2017
University Acceptances WAB Class of 2017 Asia Ecole de Commerce, Management Cornell University Saint Louis University et Gestion Aoyama Gakuin University Drexel University Sarah Lawrence College Goldsmiths, University of London National University of Singapore Duke University Savannah College of Art and Design Imperial College London Sophia University Elon University Skidmore College King’s College London Tsinghua University Emerson College Stony Brook University Leeds Beckett University University of Hong Kong Emory University Syracuse University Loughborough University Yale – NUS Fordham University University of Texas, Austin Royal Holloway, University of London Furman University Tufts University Australia School of Oriental and African Studies, George Washington University University of California, Davis Monash University University of London Grinnell College University of California, Irvine University of Melbourne Trinity College Dublin Hofstra University University of California, Los Angeles University of Sydney University College London Hult International Business School University of California, San Diego University of Western Australia Universiteit Maastricht – San Francisco University of California, Santa Barbara UNSW Australia University of Birmingham Indiana University of Bloomington University of Cincinnati University of Bristol Kalamazoo College University of Connecticut Canada University of East Anglia Lafayette College University of Denver Carleton University University of East London Lehigh College University of Illinois