A Qualitative Review of New Zealand's 2013 Level Two Risk Assessment For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Zealand Comprehensive III 16Th November – 2Nd December 2017 Trip Report
New Zealand Comprehensive III 16th November – 2nd December 2017 Trip Report Gibson’s Wandering Albatross off Kaikoura by Erik Forsyth Trip Report compiled by Tour Leader Erik Forsyth Rockjumper Birding Tours | New Zealand www.rockjumperbirding.com Trip Report – RBL New Zealand - Comprehensive III 2017 2 Daily Diary New Zealand is a must for the serious seabird enthusiast. Not only will you see a variety of albatrosses, petrels and shearwaters, but there are good chances of getting out on the high seas and finding something unusual. Seabirds dominate this tour, and views of most birds are right alongside the boat. There are also several land birds which are unique to these islands: the kiwis – terrestrial nocturnal inhabitants; the huge swamp-hen like takahe – prehistoric in its looks and movements; and then the wattlebirds (the saddlebacks and kokako) – poor flyers with short wings, which bound along the branches and on the ground. We had so many highlights on this tour, including close encounters with Little Spotted Kiwi, walk-away views of a pair of North Island Kokako, both North and South Island Saddlebacks and a pair of the impressive South Island Takahe. With many boat trips, the pelagic list was long, with Wandering, Northern and Southern Royal, Salvin’s, Black-browed, Campbell and White-capped Albatrosses, Westland, Cook’s and White- chinned Petrels, Buller’s, Flesh-footed and New Zealand (Red-breasted) Plover by Erik Forsyth Hutton’s Shearwaters, and Common Diving Petrel being a few of the highlights. Other major highlights included twelve New Zealand King Shag, a pair of Blue Duck with one chick, nine of the critically endangered Black Stilt, the rare Stitchbird, New Zealand Kaka, the entertaining Kea, range- restricted New Zealand Rockwren, the tiny Rifleman and lastly the striking Yellowhead, to name a few. -

Marlborough Sounds Wildlife Identification Guide
MARLBOROUGH SOUNDS WILDLIFE IDENTIFICATION GUIDE I NZKS operates several Salmon Farms in the Marlborough Sounds. NZKS works continuously to manage and minimise our impact on marine mammals and sea birds within the surrounding environment. An important part of our social license is to monitor wildlife activity around our farms, of which there is an abundance. Reported sightings of a specific species like King Shag can help identify patterns and trends and improve the science-based knowledge around these species and their behaviours. Information captured will also support consent requirements and future consent applications. Mammals and seabirds are instinctively attracted to the farms for shelter and food. Our focus is on minimising the risks in our daily activities and on working in harmony. This document will outline species that could visit our farms. Please use this document to help you identify any wildlife you are unsure of. Please do your utmost NOT to interact with any wild mammal or seabird, but in an event where you need to interact with them please refer to our NZKS Marine Mammal and Shark Management Plan to identify the best course of action. In the event of a wild animal dying in and around our farm, notify your manager as soon as possible. He or she will report it to the Department of Conservation (DoC) and guide you on the next course of action. Please report details of sightings or entanglements of these species to your manager: • ANY stranded/in danger wildlife • Tagged Fur seal • Leopard seal • Southern right whales SEALS • Great white II • Basking sharks • New Zealand King Shag CONTENTS Seals ............................. -

Petrelsrefs V1.1.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2017 & 2018 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney and Tom Shevlin (www.irishbirds.ie) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.1 (August 2017). Cover Main image: Bulwer’s Petrel. At sea off Madeira, North Atlantic. 14th May 2012. Picture by Killian Mullarney. Vignette: Northern Fulmar. Great Saltee Island, Co. Wexford, Ireland. 5th May 2008. Picture by Tom Shevlin. Species Page No. Antarctic Petrel [Thalassoica antarctica] 12 Beck's Petrel [Pseudobulweria becki] 18 Blue Petrel [Halobaena caerulea] 15 Bulwer's Petrel [Bulweria bulweri] 24 Cape Petrel [Daption capense] 13 Fiji Petrel [Pseudobulweria macgillivrayi] 19 Fulmar [Fulmarus glacialis] 8 Giant Petrels [Macronectes giganteus & halli] 4 Grey Petrel [Procellaria cinerea] 19 Jouanin's Petrel [Bulweria fallax] 27 Kerguelen Petrel [Aphrodroma brevirostris] 16 Mascarene Petrel [Pseudobulweria aterrima] 17 Parkinson’s Petrel [Procellaria parkinsoni] 23 Southern Fulmar [Fulmarus glacialoides] 11 Spectacled Petrel [Procellaria conspicillata] 22 Snow Petrel [Pagodroma nivea] 14 Tahiti Petrel [Pseudobulweria rostrata] 18 Westland Petrel [Procellaria westlandica] 23 White-chinned Petrel [Procellaria aequinoctialis] 20 1 Relevant Publications Beaman, M. -

Rapid Radiation of Southern Ocean Shags in Response to Receding Sea Ice 2 3 Running Title: Blue-Eyed Shag Phylogeography 4 5 Nicolas J
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.18.456742; this version posted August 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 Rapid radiation of Southern Ocean shags in response to receding sea ice 2 3 Running title: Blue-eyed shag phylogeography 4 5 Nicolas J. Rawlence1, *, Alexander T. Salis1, 2, Hamish G. Spencer1, Jonathan M. Waters1, 6 Lachie Scarsbrook1, Richard A. Phillips3, Luciano Calderón4, Timothée R. Cook5, Charles- 7 André Bost6, Ludovic Dutoit1, Tania M. King1, Juan F. Masello7, Lisa J. Nupen8, Petra 8 Quillfeldt7, Norman Ratcliffe3, Peter G. Ryan5, Charlotte E. Till1, 9, Martyn Kennedy1,* 9 1 Department of Zoology, University of Otago, Dunedin, New Zealand. 10 2 Australian Centre for Ancient DNA, University of Adelaide, South Australia, Australia. 11 3 British Antarctic Survey, Natural Environment Research Council, United Kingdom. 12 4 Instituto de Biología Agrícola de Mendoza (IBAM, CONICET-UNCuyo), Argentina. 13 5 FitzPatrick Institute of African Ornithology, Department of Biological Sciences, University 14 of Cape Town, South Africa. 15 6 CEBC-CNRS, UMR 7372, 405 Route de Prissé la Charrière, 79360 Villiers en Bois, 16 France. 17 7 Justus Liebig University, Giessen, Germany. 18 8 Organisation for Tropical Studies, Skukuza, South Africa. 19 9 School of Human Evolution and Social Change, Arizona State University, Arizona, USA. 20 21 Prepared for submission as a research article to Journal of Biogeography 22 23 * Corresponding authors: [email protected]; [email protected] 24 25 ACKNOWLEDGEMENTS 26 This work was supported with funding from the University of Otago. -

BYC-08 INF J(A) ACAP: Update on the Conservation Status
INTER-AMERICAN TROPICAL TUNA COMMISSION SCIENTIFIC ADVISORY COMMITTEE NINTH MEETING La Jolla, California (USA) 14-18 May 2018 DOCUMENT BYC-08 INF J(a) UPDATE ON THE CONSERVATION STATUS, DISTRIBUTION AND PRIORITIES FOR ALBATROSSES AND LARGE PETRELS Agreement on the Conservation of Albatrosses and Petrels (ACAP) and BirdLife International 1. STATUS AND TRENDS OF ALBATROSSES AND PETRELS Seabirds are amongst the most globally-threatened of all groups of birds, and conservation issues specific to albatrosses and large petrels led to drafting of the multi-lateral Agreement on the Conservation of Albatrosses and Petrels (ACAP). A review of the conservation status and priorities for albatrosses and large petrels was recently published in Biological Conservation (Phillips et al. 2016). There are currently 31 species listed in Annex 1 of the Agreement. Of these, 21 (68%) are classified at risk of extinction, a stark contrast to the overall rate of 12% for the 10,694 bird species worldwide (Croxall et al. 2012, Gill & Donsker 2017). Of the 22 species of albatrosses listed by ACAP, three are listed as Critically Endangered (CR), six are Endangered (EN), six are Vulnerable (VU), six are Near Threatened (NT), and one is of Least Concern (LC). Of the nine petrel species, one is listed as CR, one as EN, four as VU, one as NT and two species as LC. The population trends of ACAP species over the last twenty years (since the mid-1990s) were re-examined in 2017 by the ACAP Population and Conservation Status Working Group (PaCSWG). Thirteen ACAP species (42%) are currently showing overall population declines. -

Determining the Diet of New Zealand King Shag Using DNA Metabarcoding
Determining the diet of New Zealand king shag using DNA metabarcoding New Zealand King Shag, (Leucocarbo carunculatus) on Blumine Island, Marlborough Sounds, New Zealand in 2016 (Wikipedia commons). Aimee van der Reis & Andrew Jeffs Report Prepared For: Department of Conservation, Conservation Services Programme, Project BCBC2019-05. DOC MarineDRAFT Science Advisors Graeme Taylor and Dr Karen Middlemiss. November 2020 Reports from Auckland UniServices Limited should only be used for the purposes for which they were commissioned. If it is proposed to use a report prepared by Auckland UniServices Limited for a different purpose or in a different context from that intended at the time of commissioning the work, then UniServices should be consulted to verify whether the report is being correctly interpreted. In particular it is requested that, where quoted, conclusions given in Auckland UniServices reports should be stated in full. INTRODUCTION The New Zealand king shag (Leucocarbo carunculatus) is an endemic seabird that is classed as nationally endangered (Miskelly et al., 2008). The population is confined to a small number of colonies located around the coastal margins of the outer Marlborough Sounds (South Island, New Zealand); with surveys suggesting the population is currently stable (~800 individuals surveyed in 2020; Aquaculture New Zealand, 2020; Schuckard et al., 2015). Monitoring the colonies has become a priority and research is being conducted to better understand their population dynamics and basic ecology to improve the management of the population, particularly in relation to human activities such as fishing, aquaculture and land use (Fisher & Boren, 2012). The diet of the New Zealand king shag is strongly linked to the waters surrounding their colonies and it has been suggested that anthropogenic activities, such as marine farm structures, may displace foraging habitat that could affect the population of New Zealand king shag (Fisher & Boren, 2012). -

Picture of Bird Full Life History Info: H�P://Nzbirdsonline.Org.Nz/Species/Li�Le-Penguin
Picture Australasian gannet (Morus serrator) of bird www.nzbirdsonline.org.nz/species/australasian-gannet Introduc1on Ecology and life history The Australasian gannet is one of only three gannet species in Normal adult weight range: 2.16-2.40 kg the world. Dense breeding colonies can be found on the cliffs Moult: Gannets moult their feathers gradually and beaches of headlands on the NZ mainland and on coastal over 10-14 months. islands, as well as along the south-east coast of Australia. Breeding: August to May Majority (>85%) of the breeding populaon is found in New Egg laying between August to January Zealand. Fledglings from NZ will oen migrate to Australia and Monogamous then return to their NZ home colony aer 4-7 years to breed. Incubaon 43-53 days. Gannets are mostly coastal birds and forage on the connental Age at Fledging 91-119 days shelf. They are plunge divers and can hit the water at speeds up Age at first breeding 4-7 years. to 145km/h, therefore they do not have external nostrils. Lifespan: greater than 25 years. Instead they breath through gaps in the corner of their bills. Diet: Piscivorous CAPTURE AND HANDLING WARNING – Gannets have very sharp and serrated bill edges and a strong neck that can cause severe injuries to humans. It is ESSENTIAL to wear eye-protecon and addional hand protecon in the form of rubber welding gloves. Two people are required for procedures on gannets: one to restrain and one to perform the procedure (e.g. feeding) Personal protecve equipment (PPE): Appropriate PPE must be worn when capturing and handling oiled wildlife to prevent exposure to oil (disposable nitrile gloves, safety glasses/goggles, protecon for clothing e.g. -

Species List January 20 – February 1, 2018 | Compiled by Greg Smith
New Zealand Species List January 20 – February 1, 2018 | Compiled by Greg Smith With Greg Smith and participants Barbara, Elna, Catherine, Sanford (Sandy), Cathie, Ginger, Daniel, Muriel, George, Giulio, Lou, and Joan Species List: 113 Total Birds Seen Anatidae: Ducks, Geese, and Waterfowl (10) Greylag Goose (Anser anser) Seen early in the trip on our drive to Tiri Tiri Mantangi. Canada Goose (Branta canadensis) Usually found near most large bodies of freshwater. Black Swan (Cygnus atratus) They were also found on nearly any large body of water, usually in pairs. Paradise Shelduck (Tadorna variegata) Scattered through both of the main islands, usually in pairs or small groups. Blue Duck (Hymenolaimus malacorhynchos) We were fortunate in viewing at least twelve of these fast water ducks around Tongoriro. Australian Shoveler (Anas rhynchotis) Best looks were at birds on our drive from Picton to Kaikoura. Pacific Black Duck (Anas superciliosa) Seen well in the ponds right after our lunch near Waihu. Mallard (Anas platyrhynchos) More common than most of the other small waterfowl, very domesticated acting. Gray Teal (Anas gracilis) Seen more regularly early on in the trip as opposed to the end. New Zealand Scaup (Aythya novaeseelandiae) Very common on Great Lake Taupo and Lake Rotorua. Spheniscidae: Penguins (1) Fiordland Penguin (Eudyptes pachyrhynchus) Seen by a few of the group on Monro Beach. Odontophoridae: New World Quail (1) California Quail (Callipepla californica) Easy to find on the North Island anytime we were near brushy habitat. Phasianidae: Pheasants, Grouse, and Allies (1) Wild Turkey (Meleagris gallopavo) Seen a few times a day in paddocks that were bordered by trees on the North Island. -

Humboldt Current and the Juan Fernández Archipelago November 2014
Expedition Research Report Humboldt Current and the Juan Fernández archipelago November 2014 Expedition Team Robert L. Flood, Angus C. Wilson, Kirk Zufelt Mike Danzenbaker, John Ryan & John Shemilt Juan Fernández Petrel KZ Stejneger’s Petrel AW First published August 2017 by www.scillypelagics.com © text: the authors © video clips: the copyright in the video clips shall remain with each individual videographer © photographs: the copyright in the photographs shall remain with each individual photographer, named in the caption of each photograph All rights reserved. No part of this publication may be reproduced in any form or by any means – graphic or mechanical, including photocopying, recording, taping or information storage and retrieval systems – without the prior permission in writing of the publishers. 2 CONTENTS Report Summary 4 Introduction 4 Itinerary and Conditions 5 Bird Species Accounts 6 Summary 6 Humboldt Current and Coquimbo Bay 7 Passages Humboldt Current to Juan Fernández and return 13 At sea off the Juan Fernández archipelago 18 Ashore on Robinson Crusoe Island 26 Cetaceans and Pinnipeds 28 Acknowledgements 29 References 29 Appendix: Alpha Codes 30 White-bellied Storm-petrel JR 3 REPORT SUMMARY This report summarises our observations of seabirds seen during an expedition from Chile to the Juan Fernández archipelago and return, with six days in the Humboldt Current. Observations are summarised by marine habitat – Humboldt Current, oceanic passages between the Humboldt Current and the Juan Fernández archipelago, and waters around the Juan Fernández archipelago. Also summarised are land bird observations in the Juan Fernández archipelago, and cetaceans and pinnipeds seen during the expedition. Points of interest are briefly discussed. -

Analysis of Albatross and Petrel Distribution Within the CCAMLR Convention Area: Results from the Global Procellariiform Tracking Database
CCAMLR Science, Vol. 13 (2006): 143–174 ANALYSIS OF ALBATROSS AND PETREL DISTRIBUTION WITHIN THE CCAMLR CONVENTION AREA: RESULTS FROM THE GLOBAL PROCELLARIIFORM TRACKING DATABASE BirdLife International (prepared by C. Small and F. Taylor) Wellbrook Court, Girton Road Cambridge CB3 0NA United Kingdom Correspondence address: BirdLife Global Seabird Programme c/o Royal Society for the Protection of Birds The Lodge, Sandy Bedfordshire SG19 2DL United Kingdom Email – [email protected] Abstract CCAMLR has implemented successful measures to reduce the incidental mortality of seabirds in most of the fisheries within its jurisdiction, and has done so through area- specific risk assessments linked to mandatory use of measures to reduce or eliminate incidental mortality, as well as through measures aimed at reducing illegal, unreported and unregulated (IUU) fishing. This paper presents an analysis of the distribution of albatrosses and petrels in the CCAMLR Convention Area to inform the CCAMLR risk- assessment process. Albatross and petrel distribution is analysed in terms of its division into FAO areas, subareas, divisions and subdivisions, based on remote-tracking data contributed to the Global Procellariiform Tracking Database by multiple data holders. The results highlight the importance of the Convention Area, particularly for breeding populations of wandering, grey-headed, light-mantled, black-browed and sooty albatross, and populations of northern and southern giant petrel, white-chinned petrel and short- tailed shearwater. Overall, the subareas with the highest proportion of albatross and petrel breeding distribution were Subareas 48.3 and 58.6, adjacent to the southwest Atlantic Ocean and southwest Indian Ocean, but albatross and petrel breeding ranges extend across the majority of the Convention Area. -
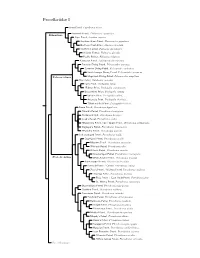
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma -

Investigating Spatiotemporal Variation in the Diet of Westland Petrel Through
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.30.360289; this version posted August 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Investigating spatiotemporal variation in the diet of Westland Petrel through 2 metabarcoding, a non-invasive technique 3 Marina Querejeta1, Marie-Caroline Lefort2,3, Vincent Bretagnolle4, Stéphane Boyer1,2 4 5 6 1 Institut de Recherche sur la Biologie de l'Insecte, UMR 7261, CNRS-Université de Tours, 7 Tours, France 8 2 Environmental and Animal Sciences, Unitec Institute of Technology, 139 Carrington Road, 9 Mt Albert, Auckland 1025, New Zealand 10 3 Cellule de Valorisation Pédagogique, Université de Tours, 60 rue du Plat d’Étain, 37000 11 Tours, France. 12 4 Centre d’Études Biologiques de Chizé, UMR 7372, CNRS & La Rochelle Université, 79360 13 Villiers en Bois, France. 14 15 16 17 18 19 Running head: Spatiotemporal variation in diet of Westland Petrel 20 21 Keywords: Conservation, metabarcoding, dietary DNA, biodiversity, New Zealand, 22 Procellaria westlandica 23 24 Abstract 25 As top predators, seabirds are in one way or another impacted by any changes in marine 26 communities, whether they are linked to climate change or caused by commercial fishing 27 activities. However, their high mobility and foraging behaviour enables them to exploit prey 28 distributed patchily in time and space. This capacity of adaptation comes to light through 29 the study of their diet.