Getting Started with Electrochemical Corrosion Measurement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

Introduction to Corrosion Your Friendly TSC Corrosion Staff
Introduction to Corrosion Your Friendly TSC Corrosion Staff: Daryl Little Ph.D. Materials Science & Engineering [email protected] 303-445-2384 Lee Sears Ph.D. Materials Science & Engineering [email protected] 303-445-2392 Jessica Torrey Ph.D. Materials Science & Engineering [email protected] 303-445-2376 Roger Turcotte, PE, CPS Materials Engineer [email protected] 303-445-2383 What is Corrosion? CORROSION IS DEFINED AS THE DETERIORATION OF A MATERIAL AND/OR ITS PROPERTIES CAUSED BY A REACTION WITH ITS ENVIRONMENT. Why is Corrosion a Major Concern? First and Most Important- Public Safety! • June 28, 1983: Mianus River Bridge, Greenwich, CT (TIME photo archive) • Northbound 3-lane section of I-95 bridge collapsed, killing 3 people • “Pin and Hanger” design was compromised when pin was displaced due to extensive corrosion of load-bearing components, amplified by salt from road maintenance. • Inadequate maintenance procedures were also cited as a contributing factor. • Subsequent to this failure, extensive inspections were conducted on this type of bridge across the country, and design modification were made to prevent catastrophic failures of this kind. First and Most Important- Public Safety! • April 28,1988, Aloha Airlines 737 Accident • 18 feet of cabin skin ripped off, resulting in the death of a flight attendant and many injuries • Attributed to corrosion fatigue Reclamation Case Study- Folsom Dam • Spillway gate No. 3 was damaged beyond repair, but no flooding occurred. • Replacement of No. 3 and repair of other gates cost ~$20 million and required 40% drainage of reservoir • Cause of failure: Increasing corrosion at the trunnion pin-hub interface raised the coefficient of friction and, therefore, the bending stress in the strut and the axial force in the brace exceeded limits. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

The Corrosion of Iron and Its Alloys
UNIVERSITY OF ILLINOIS LIBRARY Class Book Volume ^ M rlO-20M -r - 4 'f . # f- f f > # 4 Tjh- |fe ^ lINlN^ ' ^ ^ ^* 4 if i i if i^M^'M^i^ H II I li H : : \ ; . tNP* i rHt . ; ^ * 41 W 4s T »f 4 ^ -f 4. f -4- * i 4 4 : * 1- 1 4 % 3**:- ->^> A 4- - 4- + * ,,4 * 4" T '* * 4 -f- ± f * * 4- 4 -i * 4 ^ 4* -f + > * * + 1 f * 4 # * .-j* * + * ^ £ - jjR ^^MKlF^ f ^ f- + f v * IT :/^rV :,HH':,* ^ 4 * ^ 4 -f- «f f 4- 4 4 ,,rSK|M| ; * 4 # ; || II I II II f || .- -fr 4* , «g* 4 I. ^ vmiM 4- THE CORROSION OF IRON AND ITS ALLOYS BY RUSSELL SAMUEL HOWARD THESIS FOR THE DEGREE OF BACHELOR OF SCIENCE IN CHEMISTRY IN THE COLLEGE OF SCIENCE OF THE UNIVERSITY OF ILLINOIS Presented June, 1910 UNIVERSITY OF ILLINOIS June 1 1910 THIS IS TO CERTIFY THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Russel Samuel Howard ENTITLED The Corrosion of Iron and It s Alloys IS APPROVED BY ME AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE DEGREE OF Bachelor of Scienc e In Chemistry Instructor in Charge Approved: HEAD OF DEPARTMENT OF 167529 Digitized by the Internet Archive in 2013 ^ttp://archive.org/details/corrosionofironiOOhowa CORROSION OF IRON AND ITS ALLOYS. In nature everything is subject to deterioration, varying from those substances decomposed by light to those only decomposed by the strong- est agents. The deterioration of metals as effected by alloying is the sub- ject of this paper and perhaps .no subject is of more vital importance to the builder and interest to the chemist than this one of corrosion. -

INFLUENCE of Ph on the LOCALIZED CORROSION of IRON
BNL 39687 MIT/BNL-86-6 INFORMAL REPORT INFLUENCE OF pH ON THE LOCALIZED CORROSION OF IRON MIT/BNL-86-6 BNL—39687 DE87 010452 R. Webley and R. Henry Consultants: H. Isaacs and G. Cragnolino June 1986 BROOKHAVEN STATION SCHOOL OF CHEMICAL ENGINEERING PRACTICE MASSACHUSETTS INSTITUTE OF TECHNOLOGY R.O. SPROULL. DIRECTOR F.J. HRACH, ASSISTANT DIRECTOR DEPARTMENT OF APPLIED SCIENCE BROOKHAVEN NATIONAL LABORATORY ASSOCIATED UNIVERSITIES. INC. UPTON, LONG ISLAND, NEW YORK 11973 UNDER CONTRACT NO. DE-AC02-76CH00016 WITH THE UNITED STATES DEPARTMENT OF ENERGY MASTER DISTRIBUTION OF TMiS DOCUMENT IS UNLIMITED DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontrac- tors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or use- fulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency, contractor or subcontractor thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency, contractor or subcontractor thereof. ABSTRACT The influence of pH on the pitting corrosion of iron in chloride and sul- fate solutions was determined using two artificial pit apparatuses to obtain the pH near the surface of the pit bottom. -

Galvanic Corrosion
10 GALVANIC CORROSION X. G. ZHANG Teck Metals Ltd., Mississauga, Ontario, Canada A. Introduction graphite, are dispersed in a metal, or on a ship, where the B. Definition various components immersed in water are made of different C. Factors in galvanic corrosion metal alloys. In many cases, galvanic corrosion may result in D. Material factors quick deterioration of the metals but, in other cases, the D1. Effects of coupled materials galvanic corrosion of one metal may result in the corrosion D2. Effect of area protection of an attached metal, which is the basis of cathodic D3. Effect of surface condition protection by sacrificial anodes. E. Environmental factors Galvanic corrosion is an extensively investigated subject, E1. Effects of solution as shown in Table 10.1, and is qualitatively well understood E2. Atmospheric environments but, due to its highly complex nature, it has been difficult to E3. Natural waters deal with in a quantitative way until recently. The widespread F. Polarity reversal use of computers and the development of software have made G. Preventive measures great advances in understanding and predicting galvanic H. Beneficial effects of galvanic corrosion corrosion. I. Fundamental considerations I1. Electrode potential and Kirchhoff’s law I2. Analysis B. DEFINITION I3. Polarization and resistance I4. Potential and current distributions When two dissimilar conducting materials in electrical con- References tact with each other are exposed to an electrolyte, a current, called the galvanic current, flows from one to the other. Galvanic corrosion is that part of the corrosion that occurs at the anodic member of such a couple and is directly related to the galvanic current by Faraday’s law. -
1 Some Basic Concepts of Chemistry
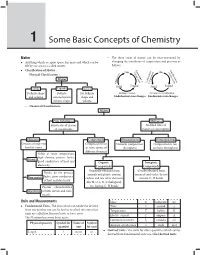
Some Basic Concepts of Chemistry 1 1 Some Basic Concepts of Chemistry Matter – The three states of matter can be inter-converted by Anything which occupies space, has mass and which can be changing the conditions of temperature and pressure as felt by our senses is called matter. follows : Ev Liq Classification of Matter : Co GasGVa ap as uefacti n po orat tion ndensa – tion Physical Classification : io riza at io on n o tion tion Matter lim ndensa or r Deposi Co Sub or Solid Liquid Solid Liquid Solids Liquids Gases Definite shape Definite No definite Melting or Fusion Freezing or Crystallization and volume volume but no shape and Endothermic state changes Exothermic state changes definite shape volume – Chemical Classification : Matter Pure Substances Mixtures Fixed ratio of masses No fixed ratio of of constituents masses of constituents Elements Compounds Homogeneous Heterogeneous Consists of only one Composed of two Uniform composition Composition is not kind of atoms or more atoms of throughout uniform throughout different elements Solids at room temperature, high density, possess lustre, Metals good conductors of heat and Organic Inorganic electricity Compounds Compounds Originally obtained from Usually obtained from Brittle, do not possess animals and plants, contain minerals and rocks, do not lustre, poor conductors Non-metals carbon and few other elements contain C–H bonds of heat and electricity like H, O, S, N, X (halogens), Possess characteristics etc. having C–H bonds Metalloids of both metals and non- metals Units and Measurements Mass m kilogram kg Fundamental Units : The units which can neither be derived Time t second s from one another nor can be further resolved into any other Temperature T kelvin K units are called fundamental units or basic units. -

Basic Concepts and Laws of Chemistry. Guidelines and Objectives for Self-Study Courses for Students in All Specialties / O.Y
THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» O.Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro 2016 THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» FACULTY OF GEOLOGICAL PROSPECTING Departament of Chemestry O. Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro NMU 2016 Svietkina O. Y. Basic concepts and laws of chemistry. Guidelines and objectives for self-study courses for students in all specialties / O.Y. Svietkina, O.B. Netyaga, G.V. Tarasova; Ministry of eduk. and sien of Ukrain, Nation. min. univer. – D . : NMU, 2016. – 20 p. Автори: О.Ю. Свєткіна, проф., д-р техн. наук (передмова, розділ 2); О.Б. Нетяга, старш. викл. (розділ 1); Г.В. Тарасова, асист. (розділ 2). Затверджено методичною комісією з галузі знань 10. Природничі науки за поданням кафедри хімії (протокол № 3 від 08.11.2016). The theoretical themes of "Basic concepts and laws of chemistry", are examples of solving common tasks, in order to consolidate the material there are presented self-study tasks for solving. Розглянуто теоретичні положення теми «Основні поняття й закони хімії», наведено приклади розв’язку типових задач з метою закріплення матеріалу, подано задачі для самостійного розв’язування. Відповідальна за випуск завідувач кафедри хімії, д-р техн. наук, проф. О.Ю. Свєткіна. Introduction Chemistry studies such form of substance motion which assumes qualitative change of matters i.e. -

Brad A. James, Ph.D., P.E., FASM
Brad A. James, Ph.D., P.E., FASM Group Vice President & Principal Engineer | Materials & Corrosion Engineering 149 Commonwealth Drive | Menlo Park, CA 94025 (650) 688-7391 tel | [email protected] Professional Profile Dr. James specializes in failure analysis, failure prevention, and integrity assessment of engineering structures and components. His specific expertise includes metallurgy, materials science, fracture, fatigue, material degradation, corrosion, life prediction, and design. In his many years of engineering experience, Dr. James has conducted thousands of failure analysis investigations on widely varying engineering structures, ranging from miniscule medical devices to power- plant components. Dr. James also helps clients from various industries prevent failures, assess the integrity of their designs or equipment, as well as interact with governmental agencies. Dr. James has special interest in fractography, fracture mechanics, wear, corrosion, embrittlement phenomena, microstructural development, heat treatment, material selection, and welding and joining. The common thread in each of Dr. James' investigations is the application of metallurgical, materials science, and engineering mechanics fundamentals to help understand and solve complex problems. Dr. James has taught several graduate-level fracture mechanics and failure analysis courses at Stanford and Santa Clara Universities. He has also taught several courses for The American Society for Materials (ASM International) involving failure analysis, design, and life prediction/validation -

(I) Determination of the Equivalent Weight and Pka of an Organic Acid
Experiment 4 (i) Determination of the Equivalent Weight and pKa of an Organic Acid Discussion This experiment is an example of a common research procedure. Chemists often use two or more analytical techniques to study the same system. These experiments can give complementary qualitative and quantitative information concerning an unknown substance. I. Titration of Acids and Bases in Aqueous Solutions The almost instantaneous reaction between acids and bases in aqueous solution produce changes in pH which one can monitor. Two techniques are useful for detecting the equivalence point: (1) colorimetry, using an acid-base color indicator - a dye which undergoes a sharp change in color in a region of pH covering the equivalence point and (2) potentiometry, using a potentiometer (pH meter) to record the sharp change at the equivalence point in the potential difference between an electrode (usually a glass electrode) and the solution whose pH is undergoing change as a result of the addition of acid or base. For example, in the case of the titration of a weak monoprotic acid HA using sodium hydroxide solution we may write: + - NaOH + HA → Na + A + H2O (4.1) Applying the law of mass action to the ionization equilibrium for the weak acid in water: + - HA + H2O H3O + A (4.2) we may write (in dilute solutions [H2O] is essentially constant) [H O+ ][A − ] 3 = K (4.3) [HA] a where Ka is the acid ionization constant (constant at any given temperature). This expression is valid for + - all aqueous solutions containing hydronium ions (H3O ), A ions, and the un-ionized molecules HA. -

The Effects and Economic Impact of Corrosion
© 2000 ASM International. All Rights Reserved. www.asminternational.org Corrosion: Understanding the Basics (#06691G) CHAPTER 1 The Effects and Economic Impact of Corrosion CORROSION is a natural process. Just like water flows to the lowest level, all natural processes tend toward the lowest possible energy states. Thus, for example, iron and steel have a natural tendency to com- bine with other chemical elements to return to their lowest energy states. In order to return to lower energy states, iron and steel frequently combine with oxygen and water, both of which are present in most natu- ral environments, to form hydrated iron oxides (rust), similar in chemi- cal composition to the original iron ore. Figure 1 illustrates the corro- sion life cycle of a steel product. Finished Steel Product Air & Moisture Corrode Steel & Form Smelting & Rust Refining Adding Giving Up Energy Energy Mining Ore Iron Oxide (Ore & Rust) Fig. 1 The corrosion cycle of steel © 2000 ASM International. All Rights Reserved. www.asminternational.org Corrosion: Understanding the Basics (#06691G) 2 Corrosion: Understanding the Basics The Definition of Corrosion Corrosion can be defined in many ways. Some definitions are very narrow and deal with a specific form of corrosion, while others are quite broad and cover many forms of deterioration. The word corrode is de- rived from the Latin corrodere, which means “to gnaw to pieces.” The general definition of corrode is to eat into or wear away gradually, as if by gnawing. For purposes here, corrosion can be defined as a chemical or electrochemical reaction between a material, usually a metal, and its environment that produces a deterioration of the material and its proper- ties. -

Introduction Engineering 360.826.4570 Nwcorrosion.Com Jeremy Hailey, P.E
Jeremy Hailey Northwest Corrosion Introduction Engineering 360.826.4570 nwcorrosion.com Jeremy Hailey, P.E. • Corrosion Engineer with 16 years experience • Started NW Corrosion Engineering 10 years ago • Focus on corrosion control system designs, troubleshooting, and some installation work • Conduct training seminars and classes for Steel Tank Institute, National Association of Corrosion Engineers, and the American Water Works Ass’n. • NACE Certified Corrosion Specialist, Cathodic Protection Specialist, and Certified Coating Inspector Jeremy Hailey Northwest Corrosion Topics of Discussion Engineering 360.826.4570 nwcorrosion.com 1. Corrosion Theory 2. Methods of Corrosion Control 3. Corrosion Protection Criteria 4. Considerations for Design of Corrosion Control Systems Jeremy Hailey Northwest Corrosion Corrosion Theory Engineering 360.826.4570 nwcorrosion.com All materials have various physical properties – Color, hardness, ductility, shear strength, ability to conduct heat, melting point, electrical potential….. Corrosion in metal occurs because of an electrical imbalance, or electrical potential difference. Much like when two beakers of water, each at a different temperature, are poured together the resulting temperature will be somewhere between the two starting temperatures. Jeremy Hailey Northwest Corrosion Requirements of a Corrosion Cell Engineering 360.826.4570 nwcorrosion.com For corrosion to occur, four individual items must be present: 1. Anode – The place where corrosion occurs; the more electronegative site 2. Cathode