Biology Can Transmutate the Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NUTRITION FACTS Maintain USDA Foods, Please Visit the FDD Web Site
OATS, WHOLE GRAIN, ROLLED, DRY Date: October 2012 PRODUCT DESCRIPTION NUTRITION INFORMATION Rolled oats are a whole grain dry cereal ½ cup of cooked rolled oats counts as 1 with no added vitamins and minerals. It ounce from the MyPlate.gov Grain Group. needs to be cooked before eating. For a 2,000-calorie diet, the daily recommendation is about 6 ounces. STORAGE Store unopened bags of oats in a cool, OTHER RESOURCES clean, dry place. www.nutrition.gov After opening, keep package tightly www.choosemyplate.gov closed. www.fns.usda.gov/fdd/ Look at the “Best if used by” or “Best by” date on the package. For further guidance on how to store and NUTRITION FACTS maintain USDA Foods, please visit the FDD Web site. Serving size: ½ cup (117g) rolled oats, cooked in water Amount Per Serving PREPARATION/COOKING Calories 80 Calories from Fat 15 Bring ½ cup water to a boil. Stir in ½ cup oats. % Daily Value* Cook 5 minutes. Total Fat 1.5g 3% Saturated Fat 0g 0% USES AND TIPS Trans Fat 0g Cook rolled oats with chopped fruit and low-fat or nonfat milk instead of water Cholesterol 0mg 0% for a filling breakfast. Sodium 5mg 0% Rolled oats can be used in a variety of baked dishes such as cookies, Total Carbohydrate 14g 5% muffins, breads, and desserts. Dietary Fiber 2g 8% Rolled oats can be used in place of Sugars 0g bread crumbs in meatloaf, or in chicken and fish patties. Protein 3g Vitamin A 0% Vitamin C 0% Calcium 2% Iron 6% *Percent Daily Values are based on a 2,000 calorie diet. -

Cereal Structure and Its Relationship to Nutritional Quality
Food Structure Volume 8 Number 1 Article 13 1989 Cereal Structure and Its Relationship to Nutritional Quality S. H. Yiu Follow this and additional works at: https://digitalcommons.usu.edu/foodmicrostructure Part of the Food Science Commons Recommended Citation Yiu, S. H. (1989) "Cereal Structure and Its Relationship to Nutritional Quality," Food Structure: Vol. 8 : No. 1 , Article 13. Available at: https://digitalcommons.usu.edu/foodmicrostructure/vol8/iss1/13 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Food Structure by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. FOOD MICROSTRUCTURE, Vol. 8 (1989), pp. 99- 113 0730- 5419/89$3 . 00+. 00 scanning Microscopy International, Chicago (AMF O'Hare) , IL 60666 USA CEREAL STRUCTURE AND ITS RELATIONSHIP TO NUTRITIONAL QUALITY S. H. Yiu Food Research Centre, Agriculture Canada, Ottawa, OntarIo, Canada KlA OC6 Abstract Introduction Factors that determine the digest1b11Hy of Cerea 1 s are good sources of carbohydrates carbohydrates and mi nera 1s 1n cerea 1 s are exa and minerals important for sustaining the energy mined . Most carbohydrates and minerals in ce and growth requirements of humans and animals. reals are structurally bound, either surrounded Cereals also contain dietary fiber. Increased by or associated with cell wall components not consumption of dietary fiber has been associated easily digested by non-ruminant animals and hu with various health benefits (Trowell , 1976; mans. Treatments such as mechanical grinding and Anderson and Chen, 197g). heat improve the digestibility of nutrients . -

Whole Or Rolled Oats for Finishing Cattle W
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange South Dakota Cattle eF eders Field Day Proceedings Animal Science Reports and Research Reports, 1980 1980 Whole or Rolled Oats for Finishing Cattle W. Vaderwert South Dakota State University L. B. Embry R. M. Luther Follow this and additional works at: http://openprairie.sdstate.edu/sd_cattlefeed_1980 Recommended Citation Vaderwert, W.; Embry, L. B.; and Luther, R. M., "Whole or Rolled Oats for Finishing Cattle" (1980). South Dakota Cattle Feeders Field Day Proceedings and Research Reports, 1980. Paper 8. http://openprairie.sdstate.edu/sd_cattlefeed_1980/8 This Report is brought to you for free and open access by the Animal Science Reports at Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in South Dakota Cattle eF eders Field Day Proceedings and Research Reports, 1980 by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. WHOLE OR ROLLED OATS FOR FINISHING CATTLE W. Vanderwert, L.B. Embry and R.M. Luther Department of Animal Science Report CATTLE CATTLE 80·7 FEEDERS DAY Summary Whole and rolled oats were compared in finishing rations for steers . On basis of feedlot performance calculated from carcass weight and 60% yield, steers fed rolled oats gained faster (10. 5%) and more efficiently (11.6%) than those fed whole oats. Carcass characteristics measured favored steers fed rolled oats and having slightly heavier carcasses . The oat rations did not compare favorab ly with normally expected performance from high-concentrate rations with corn as the maj or grain . -

2261418 Energyoatsnack Oatmeal Muesli, Apple-Cinnamon
2261418 EnergyOatSnack OatMeal Muesli, Apple-Cinnamon Sales description Whole grain rolled oats with sweeteners, protein, vitamins and minerals. white/weiß Ingredients 80% whole grain rolled oats, soy protein isolate, starch, acidifier citric acid, flavouring, calcium carbonate, sweeteners: acesulfame K, sodium cyclamate, saccharin sodium, calcium-D-pantothenate, nicotine acid amide, vitamin E, pantothenic acid, riboflavin, pyridoxine, thiamin, cobalamin.Contains gluten and soy. May contain traces of nuts. per serving / portion with 150ml water / Nutritional information per 100 g with 150ml milk (1,5%) Energy kJ kcal kJ kcal 1462 346 731 / 1031 173 / 244 Fat [g] 5,5 2,8 / 5 of which saturates[g] 1,3 0,7 / 2,2 Carbohydrates [g] 53 27 / 34 of which sugars[g] 1 0,5 / 8,3 Fibre [g] 8 4 / 4 Protein [g] 21 11 / 16 Salt [g] 0,01 0 / 0 per serving / portion per Vitamins/minerals %1 with 150ml water / %1 100 g with 150ml milk (1,5%) Vitamin E [mg] 11,4 95 5,7 / 5,7 48 / 48 Thiamin (vitamin B1) [mg] 1,8 164 0,9 / 0,9 82 / 82 Riboflavin (vitamin B2) [mg] 2 142 1 / 1 71 / 71 Niacin [mg NE] 16,8 106 8,4 / 8,4 53 / 53 Vitamin B6 [mg] 1,7g 121 0,9 / 0,9 61 / 61 Vitamin B12 [µg] 1,1 44 0,6 / 0,6 22 / 22 Pantothenic acid [mg] 5,4 90 2,7 / 2,7 45 / 45 Calcium [mg] 333 42 167 / 411 21 / 51 Phosphor [mg] 360 51 180 / 374 26 / 53 Magnesium [mg] 111 26 56 / 80 15 / 21 Iron [mg] 3,8 27 1,9 / 2,1 13 / 15 Zinc [mg] 2,4 24 1,2 / 2 12 / 20 1 per cent of the nutrient reference values according to VO (EU) no. -

Post Foodservice 1Oz Bags Sell Sheet
GRAB-N-GO FOODSERVICE 1oz Cereal Pouches from Post Available for the 22/23 School Year Featuring one of the Largest Family and Hispanic Brands - Honey Bunches of Oats NEW! 1oz Pouch Nutrition Facts FOODSERVICE 1 pouch = 1oz EQ Grain Honey Bunches of Honey Bunches Honey Bunches of Oats GRANOLA of Oats Vanilla Oats Honey Crunch 144/1oz Pouch 96/1oz Pouch 96/1oz Pouch Code: 108-84912-37482-7 Code: 108-84912-36785-0 Code: 108-84912-36786-7 Case Dimensions 18.75 x 16 x 10.38 • Cube 1.8 Case Dimensions 18.75 x 16 x 10.38 • Cube 1.8 Case Dimensions 18.75 x 16 x 10.38 • Cube 1.8 Weight 11.4 lbs • 6 TI 4 HI • 24 cs/pallet Weight 8.13 lbs • 6 TI 4 HI • 24 cs/pallet Weight 8.13 lbs • 6 TI 4 HI • 24 cs/pallet Nutrition Facts · Whole Grain Rich Nutrition Facts · Whole Grain Rich Nutrition Facts · Whole Grain Rich Serving size 1 pouch (28g) · CACFP (6g sugar) Serving size 1 pouch (28g) · CACFP (6g sugar) Serving size 1 pouch (28g) · CACFP (6g sugar) Amount per serving & Smart Snack Amount per serving & Smart Snack Amount per serving & Smart Snack Calories 120 · Kosher Calories 110 · Kosher Calories 110 · Kosher % Daily Value* % Daily Value* % Daily Value* Total Fat 3.5g 4% · No BHT Total Fat 1.5g 2% · No BHT Total Fat 1.5g 2% · No BHT Saturated Fat 0g 0% · No Nuts Saturated Fat 0g 0% · No Nuts Saturated Fat 0g 0% · No Nuts Trans Fat 0g Trans Fat 0g Trans Fat 0g Cholesterol 0mg 0% · No Artificial Flavors Cholesterol 0mg 0% · No Artificial Flavors Cholesterol 0mg 0% · No Synthetic Colors Sodium 0mg 0% Sodium 75mg 3% Sodium 75mg 3% Total Carbohydrate 21g 8% · No Synthetic Colors Total Carbohydrate 23g 8% · No Synthetic Colors Total Carbohydrate 23g 8% Dietary Fiber 2g 6% Dietary Fiber 2g 8% Dietary Fiber 2g 8% Total Sugars 6g Ingredients: Whole Grain Rolled Oats, Sugar, Total Sugars 6g Ingredients: Whole Grain Wheat, Sugar, Total Sugars 6g Ingredients: Whole Grain Wheat, Sugar, Incl. -

Who Doesn't Love Oatmeal? This Fantastic Whole Grain Is Packed With
!!!Who doesn’t love Some additions to a bowl of oatmeal are best !!!oatmeal? This when cooked right in there with the oats. !!!fantastic whole grain Consider adding... !!!is packed with fiber, • A handful of raisins, dried cranberries, protein, and other nutrients, and it makes a cherries, blueberries, or any other dried truly phenomenal breakfast. Choosing berries. oatmeal instead of a fast food breakfast • Chopped dried fruit like apricots or dates. sandwich or a breakfast pastry will cut about Step Two: Cook 20 grams of fat and over 5 teaspoons of There are two ways to prepare oatmeal -- on sugar per day. Plus, it has about 300 fewer the stove or in the microwave. Here are calories than either of those less healthful instructions for either method. options. Not totally sure how to prepare this • To make rolled oats on the stove, heat the delicious treat? Check out our tips for crafting water until boiling, add the oats, reduce to the best bowl of oatmeal. a simmer and cook until oatmeal is thick, !!! about 15 minutes. Easy as 1-2-3! • To make rolled oats in the microwave, 1. Measure your ingredients. combine your oats and water and then 2. Cook the oatmeal. microwave on high for 3-5 minutes, until 3. Add your toppings and enjoy! oatmeal is thick. Let stand for a minute or two, then stir and serve. Step One: Measure • To make steel cut oats on the stove, A plain bowl of oatmeal is made up of oats follow the stovetop instructions for rolled and water. -

Oat Basics
Give Your Family More of the Good Stuff! $hop and $ave Oat Basics d source o a goo f solub tore Wel < Compare package sizes for are le fi S l ats reat for hea be the lowest cost per ounce. O h is g rt he r hic alth aste Less < Bulk oats may cost less than w . W packaged oats. Store oats in tightly covered < containers to keep out Oats should smell faintly moisture and insects. sweet or have no aroma. Avoid I Keep In a cool, dark, dry oats that have a musty or oily cupboard. Quality is best scent. when used in 3 to 6 months. I When well packaged and stored in the freezer, they can last up to a year. Types of Oats Whole Oat grains are called Instant Oatmeal – pre-cooked Scottish Oats – the groats are groats. oat pieces have been dried and ground and broken into small Rolled Oats (Old rolled into thinner, smaller bits. They have a creamy Fashioned) – flakes. Just add hot water to porridge consistency when Whole oat prepare; can be very soft. cooked. groats are Steel Cut (Irish oats) – Oat Bran – the outer coating of steamed and whole oat groats the oat grain; very high in fiber. rolled into flakes. cut into 2 to 3 Oat Flour – A whole-grain flour Cooking time is about 5 minutes. small pieces that can be used in baking or Quick Oats – the groats are cut by steel blades. thickening. into pieces before being Cooking time is steamed longer and about 20 to 30 minutes. -

A Review of Extraction and Analysis of Bioactives in Oat and Barley and Scope for Use of Novel Food Processing Technologies
Molecules 2015, 20, 10884-10909; doi:10.3390/molecules200610884 OPEN ACCESS molecules NISSN 1420-3049 www.mdpi.com/journal/molecules Review A Review of Extraction and Analysis of Bioactives in Oat and Barley and Scope for Use of Novel Food Processing Technologies Nirupama Gangopadhyay 1,2, Mohammad B. Hossain 1, Dilip K. Rai 1,* and Nigel P. Brunton 2 1 Department of Food BioSciences, Teagasc Food Research Centre Ashtown, Dublin 15, Ireland; E-Mails: [email protected] (N.G.); [email protected] (M.B.H.) 2 Food Science and Nutrition, School of Agriculture and Food Science, University College Dublin, Dublin 4, Ireland; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +353-1-8059569; Fax: +353-1-8059550. Academic Editor: John A. Beutler Received: 7 April 2015 / Accepted: 5 June 2015 / Published: 12 June 2015 Abstract: Oat and barely are cereal crops mainly used as animal feed and for the purposes of malting and brewing, respectively. Some studies have indicated that consumption of oat and barley rich foods may reduce the risk of some chronic diseases such as coronary heart disease, type II diabetes and cancer. Whilst there is no absolute consensus, some of these benefits may be linked to presence of compounds such as phenolics, vitamin E and β-glucan in these cereals. A number of benefits have also been linked to the lipid component (sterols, fatty acids) and the proteins and bioactive peptides in oats and barley. Since the available evidence is pointing toward the possible health benefits of oat and barley components, a number of authors have examined techniques for recovering them from their native sources. -
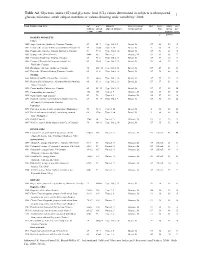
Table A2. Glycemic Index (GI)
Table A2. Glycemic index (GI) and glycemic load (GL) values determined in subjects with impaired 1 glucose tolerance, small subject numbers or values showing wide variability: 2008 Food Number and Item GI2 GI2 Subjects Reference food Ref. Serve Avail. GL3 (Glucose (Bread (type & number) & time period Size carbo- per = 100) = 100) hydrate serve g g/serve BAKERY PRODUCTS Cakes 1880 Angel food cake (Loblaw's, Toronto, Canada) 67 95±7 Type 1 & 2, 9 Bread, 3h 137 50 29 19 1881 Carrot cake, prepared with coconut flour (Philippines) 39 55±4 Type 2, 10 Bread, 3h 2 60 23 9 1882 Pound cake (Sara Lee Canada, Bramalea, Canada) 54 77±8 Type 1 & 2, 10 Bread, 3h 137 53 28 15 1883 Sponge cake, plain (Canada) 46±6 66 Normal, 5 Glucose, 2h 33 63 36 17 1884 Croissant (Food City, Toronto, Canada) 67 96±6 Type 1 & 2, 13 Bread, 3h 137 57 26 17 1885 Crumpet (Dempster's Corporate Foods Ltd., 69 98±4 Type 1 & 2, 13 Bread, 3h 137 50 19 13 Etobicoke, Canada) 1886 Doughnut, cake type (Loblaw's, Canada) 76 108±10 Type 1 & 2, 10 Bread, 3h 137 47 23 17 1887 Flan cake (Weston's Bakery, Toronto, Canada) 65 93±6 Type 1 & 2, 10 Bread, 3h 137 70 48 31 Muffins 1888 Blueberry muffin (Culinar Inc., Canada) 59 84±8 Type 1 & 2, 10 Bread, 3h 137 57 29 17 1889 Bran muffin (Culinar Inc., Grandma Martin's Muffins, 60 85±8 Type 1 & 2, 14 Bread, 2h 137 57 24 14 Aurora, Canada) 1890 Carrot muffin (Culinar Inc., Canada) 62 88±12 Type 1 & 2, 11 Bread, 3h 137 57 32 20 1891 Corn muffin, low-amylose4 102 146 Type 2, 9 Glucose, 3h 138 57 29 30 1892 Corn muffin, high-amylose4 49 70 Type 2, 9 Glucose, 3h 138 57 29 14 1893 Oatmeal, muffin, made from mix (Quaker Oats Co. -

Quaker® Instant Quick Oatmeal Variety Pack (31682)
QUAKER® INSTANT QUICK OATMEAL VARIETY PACK (31682) Case UPC 000-30000-31682-5 Package UPC 0-30000-31682-8 Case Pack 64 packets: 20 Maple Brown Sugar 16 Apple Cinnamon 12 Cinnamon Spice 16 Original Document Updated 5/15 All products are accurately labeled with the most current information however, since the ingredients are subject to change at any time, we recommend you check the label on the specific product for the most current and accurate information. QUAKER INSTANT OATMEAL MAPLE BROWN SUGAR – 1.51 oz. Nutrition Facts INGREDIENTS: WHOLE GRAIN ROLLED OATS, SUGAR, Serving Size 1 Packet (43 g) NATURAL AND ARTIFICIAL FLAVOR, SALT, CALCIUM CARBONATE, GUAR GUM, CARAMEL Amount Per Serving COLOR, NIACINAMIDE*, REDUCED IRON, VITAMIN A Calories 160 Calories from Fat 20 PALMITATE, PYRIDOXINE HYDROCHLORIDE*, RIBOFLAVIN*, % Daily Value* THIAMIN MONONITRATE*, FOLIC ACID*. Total Fat 2g 3% *ONE OF THE B VITAMINS Saturated Fat .5g 2% Trans Fat 0g Polyunsaturated Fat 0.5g Monounsaturated Fat 1.0g Kosher Status Kosher Pareve Cholesterol 0mg 0% Grain – oz. eq. 1.0 oz. eq. Sodium 260mg 11% Potassium 115mg 3% Weight of Grain 29 g Total Carbohydrate 32g 11% Document Updated 5/15 Dietary Fiber 3g 12% Soluble Fiber 1g Sugars 12g Protein 4g Vitamin A 15% Vitamin C 0% Calcium 8% Iron 15% Thiamin 15% Riboflavin 20% Niacin 20% Vitamin B6 20% Folic Acid 15% Phosphorus 10% Magnesium 10% * Percent Daily Values are based on a 2,000 calorie diet. Your daily values may be higher or lower depending on your calorie needs: Calories: 2,000 2,500 Total Fat Less than 65g 80g Sat. -

Processing of Oats and the Impact of Processing Operations on Nutrition and Health Benefits Eric A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Nutrition and Health Sciences -- Faculty Nutrition and Health Sciences, Department of Publications 2014 Processing of oats and the impact of processing operations on nutrition and health benefits Eric A. Decker University of Massachusetts na d King Abdulaziz University, [email protected] Devin J. Rose University of Nebraska–Lincoln, [email protected] Derek A. Stewart The James Hutton Institute and BioForsk Nord-Holt, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/nutritionfacpub Part of the Human and Clinical Nutrition Commons, Molecular, Genetic, and Biochemical Nutrition Commons, and the Other Nutrition Commons Decker, Eric A.; Rose, Devin J.; and Stewart, Derek A., "Processing of oats and the impact of processing operations on nutrition and health benefits" (2014). Nutrition and Health Sciences -- Faculty Publications. 31. http://digitalcommons.unl.edu/nutritionfacpub/31 This Article is brought to you for free and open access by the Nutrition and Health Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Nutrition and Health Sciences -- Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. British Journal of Nutrition (2014), 112, S58–S64 doi:10.1017/S000711451400227X q The Authors 2014 Processing of oats and the impact of processing operations on nutrition and health benefits Eric A. Decker1,2*, Devin J. Rose3 and Derek Stewart4,5 1Department of Food Science, University of Massachusetts, Amherst, MA 01003, USA 2Bioactive Natural Products Research Group, Department of Biochemistry, Faculty of Science, King Abdulaziz University, P. O. -

From the Food Bank Kitchen Cooking with Oats
From the Food Bank Kitchen Cooking with Oats January is National Oat Month! Oats are an inexpensive whole grain; this means that they do not have their bran and germ removed during processing and they have a lot of vitamins, minerals, and fiber! Oats can be prepared and used in many ways. Most oats are steamed and flattened to create rolled oats like old-fashioned oats (4-5 minutes to cook on stove) and quick oats (2-3 minutes on the stove). Steel-cut oats are also a healthy choice with a chewier, nuttier texture that can be cooked in about 5 minutes. Oats can easily be prepared on the stove or in a microwave. Besides at breakfast, there many ways to use them in other recipes. Oats are an easy way to add fiber and whole grains to your diet. On average, a serving of oatmeal and milk costs about $0.30. With so many health benefits, what’s not to love? The soluble fiber helps lower LDL (“bad”) cholesterol , may lower blood pressure, and reduce the risk of heart disease. The soluble fiber in oats also helps reduce the risk of developing type 2 diabetes and help regulate blood glucose levels in some people with diabetes by slowing the absorption of sugar into the blood. In a 1/2 cup of oats, there are 4 grams of fiber and 5 grams of protein. This much fiber and protein will keep you full longer, which may help to control weight. For more protein power, add nuts or peanut butter to oatmeal, or pair with an egg, cottage cheese, or yogurt.