Illustrated Encyclopedia of Applied and Engineering Physics, Volume 1 A–G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Engineering Physics
COLLEGE OF ENGINEERING Engineering Physics WHY ENGINEERING PHYSICS? graduates have gone on to become the CEO Engineering is about the way things work. of Eastman Kodak Co., work as acoustical Physics is about why things work the way engineers for Disney, work for the CIA, thrive they do. By combining the two, engineering in the financial sector, work in nuclear physics students are able to satisfy their engineering at Seabrook Nuclear Power Plant, curiosity and ultimately gain a deeper manage the power flow for northern New understanding of the engineering problems England, work as radiation physicists at medical centers, and become high-level UM aine’s ADVANTAGE they are working to solve. executives at SanDisk and Avis. • Professors with Ph.D. degrees, not graduate WHY STUDY ENGINEERING PHYSICS AT UMAINE? RESEARCH OPPORTUNITIES students, teach classes UMaine engineering physics was the first UMaine engineering physics students are able • State-of-the-art teaching and accredited engineering physics program in to take advantage of a myriad of research research facilities the world. Currently, it is one of only 20 programs in the U.S. and the only opportunities as undergraduates — some as • Small, student-centered accredited one in New England. e early as their freshman year. Many work classes Department of Physics and Astronomy in closely with scientists in UMaine’s Laboratory for Surface Science and Technology, which is • Opportunities to do conjunction with the College of Engineering undergraduate research offers bachelor’s and master’s degrees in internationally known for cutting-edge alongside faculty engineering physics. e curriculum research with sensors. -
B.Sc. Mechatronics Specialization: Photonic Engineering
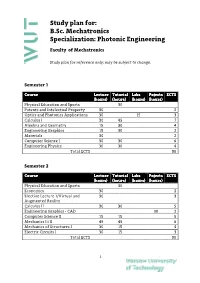
Study plan for: B.Sc. Mechatronics Specialization: Photonic Engineering Faculty of Mechatronics Study plan for reference only; may be subject to change. Semester 1 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Patents and Intelectual Property 30 2 Optics and Photonics Applications 30 15 3 Calculus I 30 45 7 Algebra and Geometry 15 30 4 Engineering Graphics 15 30 2 Materials 30 2 Computer Science I 30 30 6 Engineering Physics 30 30 4 Total ECTS 30 Semester 2 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Economics 30 2 Elective Lecture 1/Virtual and 30 3 Augmented Reality Calculus II 30 30 5 Engineering Graphics ‐ CAD 30 2 Computer Science II 15 15 5 Mechanics I i II 45 45 6 Mechanics of Structures I 30 15 4 Electric Circuits I 30 15 3 Total ECTS 30 1 Study Plan for B.Sc. Mechatronics (Spec. Photonic Engineering) Semester 3 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Elective Lecture 2/Introduction to 30 3 MEMS Calculus III 15 30 6 Mechanics of Structures II 15 15 4 Manufacturing Technology I 30 4 Fine Machine Design I 15 30 3 Electric Circuits II 30 3 Basics of Automation and Control I 30 15 4 Total ECTS 31 Semester 4 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 Foreign Language 60 4 Elective Lecture 3/Photographic 30 3 techniques in image acqusition Elective Lecture 4 30 3 /Enterpreneurship Optomechatronics 30 30 5 Electronics I 15 15 2 Electronics II 15 1 Fine Machine Design II 15 15 3 Manufacturing Technology 30 2 Metrology 30 30 4 Total ECTS 27 Semester 5 Course Lecture Tutorial Labs Pojects ECTS (hours) (hours) (hours) (hours) Physical Education and Sports 30 0 Foreign Language 60 4 Marketing 30 2 Elective Lecture 5/ Electric 30 2 2 Study Plan for B.Sc. -

Engineering Physics I Syllabus COURSE IDENTIFICATION Course
Engineering Physics I Syllabus COURSE IDENTIFICATION Course Prefix/Number PHYS 104 Course Title Engineering Physics I Division Applied Science Division Program Physics Credit Hours 4 credit hours Revision Date Fall 2010 Assessment Goal per Outcome(s) 70% INSTRUCTION CLASSIFICATION Academic COURSE DESCRIPTION This course is the first semester of a calculus-based physics course primarily intended for engineering and science majors. Course work includes studying forces and motion, and the properties of matter and heat. Topics will include motion in one, two, and three dimensions, mechanical equilibrium, momentum, energy, rotational motion and dynamics, periodic motion, and conservation laws. The laboratory (taken concurrently) presents exercises that are designed to reinforce the concepts presented and discussed during the lectures. PREREQUISITES AND/OR CO-RECQUISITES MATH 150 Analytic Geometry and Calculus I The engineering student should also be proficient in algebra and trigonometry. Concurrent with Phys. 140 Engineering Physics I Laboratory COURSE TEXT *The official list of textbooks and materials for this course are found on Inside NC. • COLLEGE PHYSICS, 2nd Ed. By Giambattista, Richardson, and Richardson, McGraw-Hill, 2007. • Additionally, the student must have a scientific calculator with trigonometric functions. COURSE OUTCOMES • To understand and be able to apply the principles of classical Newtonian mechanics. • To effectively communicate classical mechanics concepts and solutions to problems, both in written English and through mathematics. • To be able to apply critical thinking and problem solving skills in the application of classical mechanics. To demonstrate successfully accomplishing the course outcomes, the student should be able to: 1) Demonstrate knowledge of physical concepts by their application in problem solving. -

Engineering Physics I & Ii
ENGINEERING PHYSICS I & II DIPLOMA COURSE IN ENGINEERING FIRST AND SECOND SEMESTER A Publication under Government of Tamilnadu Distribution of Free Textbook Programme (NOT FOR SALE) Untouchability is a sin Untouchability is a crime Untouchability is a inhuman DIRECTORATE OF TECHNICAL EDUCATION GOVERNMENT OF TAMILNADU Government of Tamilnadu First Edition – 2015 THIRU. PRAVEEN KUMAR I.A.S Principal Secretary / Commissioner of Technical Education Directorate of Technical Education Guindy, Chennai- 600025 Dr. K.SUNDARAMOORTHY, M.E., Phd., Additional Director of Technical Education (Polytechnics) Directorate of Technical Education Guindy, Chennai- 600025 Co-ordinator Convener Er. R.SORNAKUMAR M.E., THIRU. K.SELVARAJAN M.Sc., Principal HOD (UG) / Physics Dr. Dharmambal Government Institute of Chemical Technology Polytechnic College for Women Tharamani, Chennai—113 Tharamani, Chennai—113 Reviewer Dr.K.SIVAKUMAR, M.Sc., Phd., Professor of Physics and Dean, Regional office Anna University, Madurai—7. Authors Dr.K.RAJESEKAR, M.Sc., Phd., Lecturer (UG)/ Physics Government Polytechnic College Nagercoil THIRU.G.MOHANA SUNDARAM, M.Sc.,M.Phil.,M.Ed., THIRU S.NAGARAJAN, M.Sc., HOD(UG)/Physics HOD(UG)/ Physics Central Polytechnic College Government Polytechnic College, Taramani, Chennai-113. Purasaiwakkam, Chennai –12 THIRU.N.SARAVANAN, M.Sc.,M.Phil,M.Ed., TMT.G.INDIRA, M.Sc.,M.Phil., HOD(UG)/Physics Lecturer/Physics Meenakshi Krishnan Polytechnic College Dr. Dharmambal Government Pammal, Chennai—75 Polytechnic College for Women Tharamani, Chennai—113 This book has been prepared by the Directorate of Technical Education This book has been printed on 60 G.S.M Paper Through the Tamil Nadu Text book and Educational Services Corporation ii FOREWORD With the concept of Global Village after liberalisation and globalisation, our country India has become one of the most sought after destinations by many Multi National Companies for investment. -

Tennis Court Cleaner
Robotics Overview: The purpose of this session is to learn some basics about autonomous robots. What is a robot? A robot is a specialized kind of intelligent agent. Intelligent agents represent any kind of active decision-making behavior based on achieving some goal. Robots usually mean intelligent agents that are specifically designed to sense and adjust the “real world.” A good example of an intelligent agent that is not a robot would be a smart “physicians assistant” which helps diagnose patients and keeps track of multiple drug interactions. A good example of an intelligent agent that is a robot is the Martian explorer Opportunity. Figure 1: How robots, a type of intelligent agent, typically interact with the world. Figure 1 shows how most robots are designed to interact with the world, known as the feedback control loop. The field of robotics spans many disciplines essentially because of this diagram: sensors and actuators are built using knowledge of chemistry, physics, engineering, medicine and biology. Controls are built using knowledge of computer science, mathematics, and psychology. We will mostly worry about control, or the mind here. A primitive way to implement the idea of memory is to use a state machine. If you think about how you think (a subject of psychology and artificial intelligence) your mind often has these states: when hungry seek pizza, when sleepy seek bedroom, when board call friends, etc. A state diagram formalizes this kind of state-of-mind kind of thinking which helps to design robots to behave “intelligently.” State diagrams can have many states and ways to transition from one state to another. -

Henry Ford NERS/BIOE 481 Lecture 01 Introduction
NERS/BIOE 481 Lecture 01 Introduction Michael Flynn, Adjunct Prof Nuclear Engr & Rad. Science Henry Ford Health System [email protected] [email protected] RADIOLOGY RESEARCH I.A – Imaging Systems (6 charts) A) Imaging Systems 1) General Model 2) Medical diagnosis 3) Industrial inspection NERS/BIOE 481 - 2019 2 I.A.1 - General Model – xray imaging Xrays are used to examine the interior content of objects by recording and displaying transmitted radiation from a point source. DETECTION DISPLAY (A) Subject contrast from radiation transmission is (B) recorded by the detector and (C) transformed to display values that are (D) sent to a display device for (E) presentation to the human visual system. NERS/BIOE 481 - 2019 3 I.A.2 - Medical Radiographs Traditional Modern Film-screen Digital Radiograph Radiograph NERS/BIOE 481 - 2019 4 I.A.1 - General Model – radioisotope imaging Radioisotope imaging differs from xray imaging only with repect to the source of radiation and the manner in which radiation reaches the detector DETECTION DISPLAY A B Pharmaceuticals tagged with radioisotopes accumulate in target regions. The detector records the radioactivity distribution by using a multi-hole collimator. NERS/BIOE 481 - 2019 5 I.A.2 - Medical Radisotope image Radioisotope image depicting the perfusion of blood into the lungs. Images are obtained after an intra-venous injection of albumen microspheres labeled with technetium 99m. Anterior Posterior NERS/BIOE 481 - 2019 6 I.A.3 - Industrial Radiography – homeland security Aracor Eagle High energy x-rays and a linear detector are used to scan large vehicles for border inspection NERS/BIOE 481 - 2019 7 I.A.3 - Industrial radiography – battery CT CT image of a lithium battery (Duracell CR2) “Tracking the dynamic morphology of active materials Finegan during operation of et.al., lithium batteries is Advanced essential for Science, identifying causes 2016 (3). -

A Compact Guide
A Compact Guide TO Dr. HAKIM’s COLLECTION OF ANTIQUE X-RAY TUBES AND ACCESSORIES October 2014 INDEX Page Number Crookes Tubes……………………………... 1 Cold Cathode Ion X-Ray Tubes…………… 2-3-4-5 Cold Cathode Rectifier Valves…………….. 6 Air-Cooled Hot Cathode ( Coolidge ) X-Ray Tubes……………………………………….. 7-8-9 Oil-Immersed Hot-Cathode Fixed Anode Tubes, Unusual Tubes, and Tubes with Special Functions ………………………….. 9-10-11 Air-Cooled Kenotrons ( Rectifier Valves )….. 13-14 Oil-Immersed Kenotrons ( Rectifier Valves ).. 15-16-17 Radiology-Related Items…………………... 18-19-20- 21-22 1 Crookes Tubes Early basic type Crookes tube This is a 20 th Century replica of the Crookes tube with which W.C.Roëntgen was experimenting when he discovered an unknown type of rays which he called “x”rays Experimental multi-electrode Crookes tube (One cathode and three anodes ). Early 20 th Century Adjustable vacuum multi-electrode discharge tube, with two cathodes ( in the long arms ), one flat anode on top of the spherical bulb, and an anti-cathode in the centre of the sphere, covered with platinum foil Experimental Crookes tube for studying the deflection of cathode rays in a magnetic field 2 Cold Cathode Ion X-Ray Tubes The “ Jackson Tube ”, the earliest two electrode “Focus X-Ray Tube”, (concave aluminum cathode and flat platinum anti-cathode ) 1896 Late 19 th Century or early 20 th Century three electrode focus x-ray tube (Aluminium concave cathode, flat aluminium anode, and thin platinum anti-cathode). No Regeneration. Early 20 th Century x-ray tube similar in conception to the tube above, but with an aluminium rod anode, and of a more recent make No regeneration Very rare late 19 th Century 3 electrode cylindrical x-ray tube. -

Engineering Physics Dept: Engineering and Physics Major: Electrical Engineering College: Mathematics and Science Degree: Master of Science (M.S.) Major Code: 6633
University of Central Oklahoma Graduate Catalog 2021-2022 Program: Engineering Physics Dept: Engineering and Physics Major: Electrical Engineering College: Mathematics and Science Degree: Master of Science (M.S.) Major Code: 6633 Engineering Physics - Electrical Engineering, M.S. This major is designed so that its graduates can enter industry/government as practicing engineers or pursue research and teaching careers with government and/or academic institutions. The major also provides advanced study in electrical engineering, with emphasis in communications and signal processing, for students who intend to pursue a Ph.D. degree in Electrical Engineering or related fields. Graduate Advisor: Dr. Yuhao Jiang Graduate Studies. Email: [email protected] Students applying for a Master Degree through the Accelerated Office: STEM 242 BS/MS Degree Program must submit the following items to the Phone: 405 - 974 - 5472 Engineering Physics Department Accelerated Program Admissions Committee: Admission Requirements • Application for admission received by March 5 in the spring semester of the junior year. Submit the following items to: • Official copies of transcripts from each institution attended. Jackson College of Graduate Studies Transcripts must show: 100 N. University Drive, NUC 404 ◦ The applicant is a UCO Electrical Engineering major; Edmond, OK 73034 ◦ A minimum overall GPA of 3.00; • Online application for admission (www.uco.edu/graduate/). ◦ A minimum GPA of 3.00 in all Engineering and Physics • Official copies of undergraduate and graduate transcripts from courses specified in the junior year for their major. Grades each institution attended with all degrees posted. All transcripts for engineering courses taken in the spring semester of the must be from accredited institutions. -

Atomic Theory - Review Sheet
Atomic Theory - Review Sheet You should understand the contribution of each scientist. I don't expect you to memorize dates, but I do want to know what each scientist contributed to the theory. Democretus - 400 B.C. - theory that matter is made of atoms Boyle 1622 -1691 - defined element Lavoisier 1743-1797 - pioneer of modern chemistry (careful and controlled experiments) - conservation of mass (understand his experiment and compare to the lab "Does mass change in a chemical reaction?") Proust 1799 - law of constant composition (or definite proportions) - understand this law and relate it to the Hydrogen and Oxygen generation lab Dalton 1808 - Understand the meaning of each part of Dalton's atomic theory. - You don't have to memorize the five parts, just understand what they mean. - You should know which parts we now know are not true (according to modern atomic theory). Black Box Model Crookes 1870 - Crookes tube - Know how this is constructed (Know how cathode rays are generated and what they are.) J.J. Thomsen - 1890 - How did he expand on Crookes' experiment? - discovered the electron - negative particles in all of matter (all atoms) - must also be positive parts Lord Kelvin - near Thomsen's time Plum Pudding Model - plum pudding model Henri Becquerel - 1896 - discovered radioactivity of uranium Marie Curie - 1905 - received Nobel prize (shared with her husband Pierre Curie and Henri Bequerel) for her work in studying radiation. - Name the three kinds of radiation and describe each type. Rutheford - 1910 - Understand the gold foil experiment - How was it set up? - What did it show? - Discovered the proton Hard Nucleus Model - new model of atom (positively charged, very dense nucleus) Niels Bohr - 1913 - Bohr model of the atom - electrons in specific orbits with specific energies James Chadwick - 1932 - discovered the electron Modern version of atom - 1950 Bohr Model - similar nucleus (protons + neutrons) - electrons in orbitals not orbits Bohr Model Understand isotopes, atomic mass, mass #, atomic #, ions (with neutrons) Nature of light - wavelength vs. -

The Cathode Ray Tube 1
Kendall Dix The Cathode Ray Tube 1 PHYSICS II HONORS PROJECT THE CATHODE RAY TUBE BY: KENDALL DIX 001-H ID: 010363995 Kendall Dix The Cathode Ray Tube 2 Introduction !The purpose of this construction project was the replication of Crookes tube, or the cathode ray tube. I was inspired by the historical and modern significance of the cathode ray. A modification of Crookes tube evolved into the first televisions. Although these are being phased out, their importance is undeniable. In modern times the basic cathode ray is not used for anything but demonstrating gasses conducting electricity. How it should work !A Crookes tube is constructed by applying a voltage from a few kV to 100 kV between a cathode and anode (Gilman). Although the Crookes tube requires an evacuation of gas, it must not be complete. The remaining gas is crucial to the process. The cathode (negative side) is induced to emit electrons by naturally occurring positive ions in the air (Townsend). The positive ions are attracted to the negative charge of the cathode. They collide with other air particles and strip off electrons, creating more positive ions. The positive ions collide with the cathode so powerfully that they knock off the excess electrons. These electrons are immediately attracted to the anode and begin accelerating toward it. Because Crookes tube is a cold cathode (no heat applied), the cathode requires the impact of the ions to knock off electrons. The maximum vacuum or minimum gas pressure to begin the chain reaction of ions in the gas is about 10-6 atm (Thompson). -

2008 NSF-Sponsored Report: the Future of Materials Science And
THE FUTURE OF MATERIALS SCIENCE AND MATERIALS ENGINEERING EDUCATION A report from the Workshop on Materials Science and Materials Engineering Education sponsored by the National Science Foundation September 18-19, 2008 in Arlington, VA TABLE OF CONTENTS Summary . 5. Summary of the Recommendations . 9. Public Education and Outreach Recommendations . 9. Kindergarten through 12th Grade (K-12) Education Recommendations . 9. Undergraduate Education Recommendations . 10. Graduate Education Recommendations . 10. 1. Public Education and Outreach . 13. 1 .1 Introduction . 13. 1 .2 What Does the Public Know? . 13. 1 .3 What Should the Public Know? . 14. 1 .4 How Does the Public Learn About Materials Science and Materials Engineering? . 17. 1 5. How Can the Materials Community Promote Learning Using Informal Science Education? . 20. 1 .6 What is the Impact of Outreach Activities on the Career Development of Faculty? . 21. 1 .7 Recommendations . 22. 2. Kindergarten Through 12th Grade (K-12) Education . 23. 2 .1 Introduction . 23. 2 .2 Materials Education Standards and Curricula for K-12 Students . 24. 2 .3 Professional Development of K-12 Teachers . 27. 2 .4 Career Awareness for K-12 Students . 28. 2 .5 Recommendations . 29. 3. Undergraduate Education . 31. 3 .1 Introduction . 31. 3 .2 Curriculum Development . 32. 3 .3 Recruiting and Retaining Students in MSME . 34. 3 .4 Recommendations . 35. 4. Graduate Education . 37. 4 .1 Introduction . 37. 4 .2 Course Curriculum . 39. 4 .3 Interdisciplinary Training . 41. 4 .4 Career Preparation . 43. 4 .5 Recommendations . 44. 5. Cross-Cutting Theme: Use of Information Technology in MSME Education and Research . 45. 6. Workshop Program . 47. 7. -

Engineering Physics a Guide for Undergraduate Majors
Engineering Physics A Guide for Undergraduate Majors Last modified Fall 2015 This guide applies to students entering the program after August 2015. Students admitted prior to this should continue to follow the Undergraduate Student guide in effect when they entered the program. They may petition the department to select features of the new curriculum. Administered by the Department of Engineering Physics 153 Engineering Research Building, 1500 Engineering Drive, Madison, WI 53706-1609 Phone: (608) 263-1646, Fax: (608) 263-7451, Internet: www.engr.wisc.edu/ep/ Introduction The Engineering Physics Undergraduate Program (EP) is administered by the Department of Engineering Physics. The Department Office is room 153 Engineering Research Building (ERB). The Department Chair’s office is also in room 153 ERB. The department also administers the Engineering Mechanics undergraduate and graduate programs (EM) as well as the Nuclear Engineering undergraduate (NE) and the Nuclear Engineering and Engineering Physics graduate programs (NEEP). This guide is intended to provide Engineering Physics undergraduate students with information that will facilitate their studies at the University of Wisconsin-Madison. In addition to this guide, you should consult the Undergraduate Catalog (www.pubs.wisc.edu/ug/engr_engphys.htm) for requirements, objectives, and course descriptions. You may also find the resources page at the UW website helpful https://www.admissions.wisc.edu/ The Engineering Physics Department web site is www.engr.wisc.edu/ep.html. There are links to the Engineering Physics, Engineering Mechanics, and Nuclear Engineering programs. Updated curriculum and course information is included on the department website. The College of Engineering website (www.engr.wisc.edu/ep.html) also provides information for engineering students.