3.2.4 Sertraline and Nortriptyline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Efficacy of Treatments for Patients with Obsessive-Compulsive Disorder: a Systematic Review
REVIEW Efficacy of treatments for patients with obsessive-compulsive disorder: A systematic review Yun-Jung Choi, PhD, RN, PMHNP (Lecturer) Keywords Abstract Systematic review; obsessive-compulsive disorder; efficacy of medication. Purpose: This systematic review examines the efficacy of pharmacological therapy for obsessive-compulsive disorder (OCD), addressing two major issues: Correspondence which treatment is most effective in treating the patient’s symptoms and which Yun-Jung Choi, PhD, RN, PMHNP, Red Cross is beneficial for maintaining remission. College of Nursing, 98 Saemoonan-Gil, Data sources: Seven databases were used to acquire articles. The key words Jongno-Gu, Seoul 110-102, Korea; used to search for the relative topics published from 1996 to 2007 were Tel: +82-2-3700-3676; fax: +82-2-3700-3400; ‘‘obsessive-compulsive disorder’’ and ‘‘Yale-Brown obsession-compulsion E-mail: [email protected] scale.’’ Based on the inclusion and exclusion criteria, 25 studies were selected Received: August 2007; from 57 potentially relevant studies. accepted: March 2008 Conclusions: The effects of treatment with clomipramine and selective sero- tonin reuptake inhibitors (SSRIs: fluvoxamine, sertraline, fluoxetine, citalo- doi:10.1111/j.1745-7599.2009.00408.x pram, and escitalopram) proved to be similar, except for the lower adherence rate in case of clomipramine because of its side effects. An adequate drug trial involves administering an effective daily dose for a minimum of 8 weeks. An augmentation strategy proven effective for individuals refractory to monother- apy with SSRI treatment alone is the use of atypical antipsychotics (risperidone, olanzapine, and quetiapine). Implications for practice: Administration of fluvoxamine or sertraline to patients for an adequate duration is recommended as the first-line prescription for OCD, and augmentation therapy with risperidone, olanzapine, or quetiapine is recommended for refractory OCD. -

Impact of CYP2C19 Genotype on Sertraline Exposure in 1200 Scandinavian Patients
www.nature.com/npp ARTICLE Impact of CYP2C19 genotype on sertraline exposure in 1200 Scandinavian patients Line S. Bråten 1,2, Tore Haslemo1,2, Marin M. Jukic3,4, Magnus Ingelman-Sundberg 3, Espen Molden1,5 and Marianne K. Kringen1,2 Sertraline is an (SSRI-)antidepressant metabolized by the polymorphic CYP2C19 enzyme. The aim of this study was to investigate the impact of CYP2C19 genotype on the serum concentrations of sertraline in a large patient population. Second, the proportions of patients in the various CYP2C19 genotype-defined subgroups obtaining serum concentrations outside the therapeutic range of sertraline were assessed. A total of 2190 sertraline serum concentration measurements from 1202 patients were included retrospectively from the drug monitoring database at Diakonhjemmet Hospital in Oslo. The patients were divided into CYP2C19 genotype-predicted phenotype subgroups, i.e. normal (NMs), ultra rapid (UMs), intermediate (IMs), and poor metabolisers (PMs). The differences in dose-harmonized serum concentrations of sertraline and N-desmethylsertraline-to-sertraline metabolic ratio were compared between the subgroups, with CYP2C19 NMs set as reference. The patient proportions outside the therapeutic concentration range were also compared between the subgroups with NMs defined as reference. Compared with the CYP2C19 NMs, the sertraline serum concentration was increased 1.38-fold (95% CI 1.26–1.50) and 2.68-fold (95% CI 2.16–3.31) in CYP2C19 IMs and PMs, respectively (p < 0.001), while only a marginally lower serum concentration (−10%) was observed in CYP2C19 UMs (p = 0.012). The odds ratio for having a sertraline concentration above the therapeutic reference range was 1.97 (95% CI 1.21–3.21, p = 0.064) and 8.69 (95% CI 3.88–19.19, p < 0.001) higher for IMs and PMs vs. -

ZOLOFT® 50 Mg and 100 Mg Tablets
NEW ZEALAND DATA SHEET 1. PRODUCT NAME ZOLOFT® 50 mg and 100 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 50 mg tablet contains sertraline hydrochloride equivalent to 50 mg sertraline. Each 100 mg tablet contains sertraline hydrochloride equivalent to 100 mg sertraline. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM ZOLOFT 50 mg tablets: white film-coated tablets marked with the Pfizer logo on one side and “ZLT” scoreline “50” on the other. Approximate tooling dimensions are 1.03 cm x 0.42 cm x 0.36 cm. ZOLOFT 100 mg tablets: white film-coated tablets marked with the Pfizer logo on one side and “ZLT-100” or “ZLT 100” on the other. Approximate tooling dimensions are 1.31 cm x 0.52 cm x 0.44 cm. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Adults ZOLOFT is indicated for the treatment of symptoms of depression, including depression accompanied by symptoms of anxiety, in patients with or without a history of mania. Following satisfactory response, continuation with ZOLOFT therapy is effective in preventing relapse of the initial episode of depression or recurrence of further depressive episodes. ZOLOFT is indicated for the treatment of obsessive compulsive disorder (OCD). Following initial response, sertraline has been associated with sustained efficacy, safety and tolerability in up 2 years of treatment of OCD. ZOLOFT is indicated for the treatment of panic disorder, with or without agoraphobia. ZOLOFT is indicated for the treatment of post-traumatic stress disorder (PTSD). ZOLOFT is indicated for the treatment of social phobia (social anxiety disorder). -

Sertraline and Venlafaxine-Induced Nocturnal Enuresis
Case Report DOI: 10.5455/bcp.20140304091103 Sertraline and Venlafaxine-Induced Nocturnal Enuresis Inci Meltem Atay1, Gulin Ozdamar Unal2 ABS TRACT: Sertraline and venlafaxine-induced nocturnal enuresis Nocturnal enuresis is defined as the involuntary discharge of urine after the age of expected continence that occurs during sleep at night. Although there are a few reports in adults for nocturnal enuresis associated 1Assist. Prof., 2M.D., Suleyman Demirel University, School of Medicine, Department with serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), the of Psychiatry, Isparta - Turkey mechanism or frequency of this side effect have not been identified yet. We report here a case of nocturnal Corresponding author: Dr. İnci Meltem Atay enuresis associated with both sertraline and venlafaxine in different major depressive episodes in an adult Süleyman Demirel Universitesi, Tıp Fakültesi, Psikiyatri Anabilim Dalı, Isparta - Türkiye patient that resolves after the discontinuation of the medications and continuation with escitalopram. To our E-ma il add ress: knowledge, in literature there have been no reports about nocturnal enuresis caused by those two agents in [email protected] the same patient. This case is discussed in detail for the recurrence of nocturnal enuresis, the importance of Date of submission: detailed assessment of even rare side effects and for their possible mechanisms. February 11, 2014 Date of acceptance: March 04, 2014 Keywords: nocturnal enuresis, venlafaxine, sertraline, depression Declaration of interest: I.M.A, G.O.U.: The authors reported no Klinik Psikofarmakoloji Bulteni - Bulletin of Clinical Psychopharmacology 2015;25(3):287-90 conflict of interest related to this article. INTRODUCTION side effect1-6. -
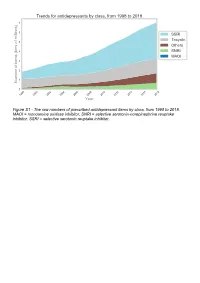
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

NORPRAMIN® (Desipramine Hydrochloride Tablets USP)
NORPRAMIN® (desipramine hydrochloride tablets USP) Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of NORPRAMIN or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. NORPRAMIN is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.) DESCRIPTION NORPRAMIN® (desipramine hydrochloride USP) is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz[bƒ]azepine-5-propanamine,10,11-dihydro-N-methyl-, monohydrochloride. 1 Reference ID: 3536021 Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: acacia, calcium carbonate, corn starch, D&C Red No. 30 and D&C Yellow No. 10 (except 10 mg and 150 mg), FD&C Blue No. 1 (except 25 mg, 75 mg, and 100 mg), hydrogenated soy oil, iron oxide, light mineral oil, magnesium stearate, mannitol, polyethylene glycol 8000, pregelatinized corn starch, sodium benzoate (except 150 mg), sucrose, talc, titanium dioxide, and other ingredients. -

Current P SYCHIATRY
Current p SYCHIATRY N ew Investigators Tips to manage and prevent discontinuation syndromes Informed tapering can protect patients when you stop a medication Sriram Ramaswamy, MD Shruti Malik, MBBS, MHSA Vijay Dewan, MD Instructor, department of psychiatry Foreign medical graduate Assistant professor Creighton University Department of psychiatry Omaha, NE University of Nebraska Medical Center Omaha, NE bruptly stopping common psychotropics New insights on psychotropic A —particularly antidepressants, benzodi- drug safety and side effects azepines, or atypical antipsychotics—can trigger a discontinuation syndrome, with: This paper was among those entered in the 2005 • rebound or relapse of original symptoms Promising New Investigators competition sponsored • uncomfortable new physical and psycho- by the Neuroleptic Malignant Syndrome Information Service (NMSIS). The theme of this year’s competition logical symptoms was “New insights on psychotropic drug safety and • physiologic withdrawal at times. side effects.” To increase health professionals’ awareness of URRENT SYCHIATRY 1 C P is honored to publish this peer- the risk of these adverse effects, this article reviewed, evidence-based article on a clinically describes discontinuation syndromes associated important topic for practicing psychiatrists. with various psychotropics and offers strategies to NMSIS is dedicated to reducing morbidity and anticipate, recognize, and manage them. mortality of NMS by improving medical and psychiatric care of patients with heat-related disorders; providing -

And N-Demethylation of Venlafaxine in Vitro by Human Liver Microsomes
O- and N-demethylation of Venlafaxine In Vitro by Human Liver Microsomes and by Microsomes from cDNA-Transfected Cells: Effect of Metabolic Inhibitors and SSRI Antidepressants Steven M. Fogelman, Jürgen Schmider, Karthik Venkatakrishnan, Lisa L. von Moltke, Jerold S. Harmatz, Richard I. Shader, and David J. Greenblatt The biotransformation of venlafaxine (VF) into its two to the formation of NDV for all four livers tested. major metabolites, O-desmethylvenlafaxine (ODV) and Parameters determined by applying a single-enzyme model 5 / / 5 N-desmethylvenlafaxine (NDV) was studied in vitro with were Vmax 2.14 nmol min mg protein, and Km 2504 human liver microsomes and with microsomes containing mM. Ketoconazole was a potent inhibitor of NDV individual human cytochromes from cDNA-transfected production, although its inhibitory activity was not as great human lymphoblastoid cells. VF was coincubated with as observed with pure 3A substrates. NDV formation was selective cytochrome P450 (CYP) inhibitors and several also reduced by 42% by a polyclonal rabbit antibody against selective serotonin reuptake inhibitors (SSRIs) to assess rat liver CYP3A1. Studies using expressed cytochromes their inhibitory effect on VF metabolism. Formation rates showed that NDV was formed by CYP2C9, 22C19, and for ODV incubated with human microsomes were 23A4. The highest intrinsic clearance was attributable to consistent with Michaelis-Menten kinetics for a single- CYP2C19 and the lowest to CYP3A4. However the high in enzyme mediated reaction with substrate inhibition. Mean vivo abundance of 3A isoforms will magnify the 5 parameters determined by non-linear regression were: Vmax importance of this cytochrome. Fluvoxamine (FX), at a / / 5 m m 0.36 nmol min mg protein, Km 41 M, and Ks 22901 concentration of 20 M, decreased NDV production by m M (Ks represents a constant which reflects the degree of 46% consistent with the capacity of FX to inhibit CYP3A, substrate inhibition). -

Determination of Sertraline and Its Metabolite by High-Pressure Liquid Chromatography in Plasma
ACADEMIA ROMÂNĂ Rev. Roum. Chim., Revue Roumaine de Chimie 2015, 60(5-6), 543-548 http://web.icf.ro/rrch/ DETERMINATION OF SERTRALINE AND ITS METABOLITE BY HIGH-PRESSURE LIQUID CHROMATOGRAPHY IN PLASMA Nazan YUCE-ARTUN,a Erguvan Tuğba ÖZEL KIZIL,b Bora BASKAK,b Halise Devrimci ÖZGÜVEN,b Yalçın DUYDUc and Halit Sinan SUZENc,* aBiotechnology Institute, Ankara University, Golbasi, Ankara, Turkey bDepartment of Psychiatry, School of Medicine, Ankara University, Dikimevi, Ankara, Turkey cDepartment of Toxicology, Faculty of Pharmacy, Ankara University, Tandogan, Ankara, Turkey Received November 10, 2014 A fast, simple and sensitive high-pressure liquid chromatography (HPLC) method with UV detection was developed for frequently prescribed antidepressant, sertraline (SERT) and its main B metabolite N-desmethylsertraline (DSERT), in human plasma. SERT and DSERT were extracted by an optimized solid phase chromatographic (SPE) method using C-18 cartridges and DSE SER Clomipramine was used as external standard (ES). The analytes ES were separated on C18, 4.6 mm × 150 mm, 5 µm column at 50 °C with a mobile phase of 45% acetonitrile + 55% NaH2PO4 at a flow rate of 0.4 mL/min. Detector responses monitored at 4 different wave-lengths; 200-205-210-215 nm. The method proved to be rapid and effective for the plasma sample analyses of therapeutic drug monitoring for sertraline treated patients. INTRODUCTION* depression, panic disorder, generalised anxiety disorder, and social phobia.2 Like other SSRIs, it High rates of poor compliance, considerable has a wide therapeutic index and seems to be better genetic variability in metabolism, and the clinical tolerated than tricyclic antidepressants.3 The drug heterogeneity of depression are the main problems is slowly absorbed with a time to peak plasma for the practical application of selective serotonin concentration of approximately 4–8 h and an reuptake inhibitors (SSRI).1 Therapeutic drug elimination half life of 22–35 h. -

Hyponatremia and Psychotropic Drugs
DOI: 10.5772/intechopen.79029 ProvisionalChapter chapter 2 Hyponatremia and Psychotropic Drugs MireiaMireia Martínez Cortés Martínez Cortés and Pedro Gurillo MuñozPedro Gurillo Muñoz Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.79029 Abstract Given the widespread use of psychotropic drugs in the population, it’s important to consider hyponatremia as an avoidable and reversible adverse effect and include the detection of high-risk subjects to establish safer medications, as well as early detection measures in routine clinical practice. Although hyponatremia has been especially associ- ated with serotonergic antidepressants (SSRIs), there is also an elevated risk with tricy- clics, duals and heterocyclic antidepressants, due to the different mechanisms of action at the renal tubular level and the release of ADH. Hyponatremia secondary to tricyclics with slow CYP2D6 metabolizers have higher plasma concentrations of antidepressants metabolized by CYP2D6. Hyponatremia secondary to SSRIs appears in the first week of treatment, it is “not dose-dependent” and normalization of natremia occurs between 2 and 20 days after stopping the medication. Bupropion, trazodone, mianserin, reboxetine and agomelatine are a safe alternative. Also antiepileptics have been related to hypona- tremia. Both typical and atypical antipsychotics have been exposed to an increased risk of hyponatremia, even after adjusted factors such as age, sex and comorbidity. Other factors that favor the onset of hyponatremia act synergistically with psychotropic drugs, such as: advanced age, female sex, concomitant diuretic intake, low body weight and low sodium levels; NSAID, ACEIs, and warm. Keywords: hyponatremia, antipsychotic, antidepressant, antiepileptic, psychotropic drugs 1. Introduction Hyponatremia is the most frequent hydroelectrolytic disorder in clinical practice, both in hospital and outpatient settings [1]. -

Fatal Toxicity of Antidepressant Drugs in Overdose
BRITISH MEDICAL JOURNAL VOLUME 295 24 OCTOBER 1987 1021 Br Med J (Clin Res Ed): first published as 10.1136/bmj.295.6605.1021 on 24 October 1987. Downloaded from PAPERS AND SHORT REPORTS Fatal toxicity of antidepressant drugs in overdose SIMON CASSIDY, JOHN HENRY Abstract dangerous in overdose, thus meriting investigation of their toxic properties and closer consideration of the circumstances in which A fatal toxicity index (deaths per million National Health Service they are prescribed. Recommendations may thus be made that prescriptions) was calculated for antidepressant drugs on sale might reduce the number offatalities. during the years 1975-84 in England, Wales, and Scotland. The We used national mortality statistics and prescription data tricyclic drugs introduced before 1970 had a higher index than the to compile fatal toxicity indices for the currently available anti- mean for all the drugs studied (p<0-001). In this group the depressant drugs to assess the comparative safety of the different toxicity ofamitriptyline, dibenzepin, desipramine, and dothiepin antidepressant drugs from an epidemiological standpoint. Owing to was significantly higher, while that ofclomipramine, imipramine, the nature of the disease these drugs are particularly likely to be iprindole, protriptyline, and trimipramine was lower. The mono- taken in overdose and often cause death. amine oxidase inhibitors had intermediate toxicity, and the antidepressants introduced since 1973, considered as a group, had significantly lower toxicity than the mean (p<0-001). Ofthese newer drugs, maprotiline had a fatal toxicity index similar to that Sources ofinformation and methods of the older tricyclic antidepressants, while the other newly The statistical sources used list drugs under their generic and proprietary http://www.bmj.com/ introduced drugs had lower toxicity indices, with those for names. -

Heat Related Illness in Psychotropic Medication Users
Common psychotropic medications which Prevention of Heat can impair your response to heat Related Illness Trade Name Generic Name Abilify aripiprazole During periods of high temperature (85º Asendin amoxapine and above) and humidity, there are things Artane trihexyphenidyl everyone, particularly people at high risk, Aventil, Pamelor nortriptyline should do to lessen the chances of heat Clozaril clozapine illness. Cogentin benztropine Compazine prochlorperazine ¾ Try to stay cool. Desyrel trazodone • Stay in air conditioned areas if Elavil, Limbitrol, possible. If you do not have air Triavil amitriptyline conditioning at home, go to a Eskalith, Lithobid, shopping mall or public library. Lithonate lithium • Keep windows shut and draperies, Geodon ziprasidone shades, or blinds drawn during the Haldol haloperidol heat of the day. Loxitane loxapine • Open windows in the evening or Ludiomil maprotiline night hours when the air outside is Mellaril thioridazine Heat Related Illness cooler. Moban molindone • Move to cooler rooms during the Navane thiothixene in heat of the day. Norpramin desipramine Psychotropic ¾ Avoid overexertion and outdoor Phenergan promethazine activity, particularly during warmer Prolixin fluphenazine Medication Users periods of the day. Risperdal risperidone ¾ Apply sunscreen and lotion as needed. Serentil mesoridazine Seroquel quetiapine ¾ Drink plenty of fluids (avoid coffee, tea, and alcohol). Sinequan doxepin ¾ Dress in loose fitting, light colored Stelazine trifluoperazine clothing. Wear a hat, sunglasses, and Thorazine chlorpromazine other protective clothing. Tofranil imipramine ¾ Take a cool shower or bath. Trilafon perphenazine ¾ Lose weight if you are overweight. Wellbutrin buproprion ¾ Eat regular meals to ensure that you Zyprexa olanzapine Ohio Department of Mental Health have adequate salt and fluids. *Note: This is not an all inclusive list.