Phytoplankton Dynamics of the River Nene, England
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mick George Ltd for the Proposal to Extract 1.95 Million Tonnes of Limestone and Import a Similar Volume of Inert Fill to Achieve a Suitable Landform Upon Restoration
Ringstead Grange Quarry Ringstead, Northamptonshire VOL I ENVIRONMENTAL STATEMENT (Written Text and Plans) Proposals for Extraction of Limestone and Importation of Suitable Inert Fill MARCH 2012 Ringstead Grange Extraction of limestone and importation of inert Environmental Statement March 2012 CONTENTS Non-Technical Summary 1.Introduction ................................................................................................................. 1 1.1. Aims of the Environmental Statement .................................................................................... 1 1.2. Scoping Response ................................................................................................................... 2 1.3. Summary of Proposals ............................................................................................................ 2 1.4. Methodology Framework ....................................................................................................... 4 1.5. Planning History ..................................................................................................................... 5 1.6. The Applicant Company ......................................................................................................... 7 1.7. Sustainable Development and Biodiversity ............................................................................ 8 2.Statement of Community Involvement ..................................................................... 10 3.Site and Environs ....................................................................................................... -

Assessment Appendices Biodiversity Character
BIODIVERSITY CHARACTER ASSESSMENT APPENDICES CONTENTS APPENDIX 1 Datasets used in the Northamptonshire Biodiversity Character Assessment ................................................................. 03 APPENDIX 2 Natural Areas in Northamptonshire .............................................................................................................................................. 04 Natural Area 44. Midlands Clay Pastures ....................................................................................................................................... 04 Natural Area 45. Rockingham Forest .............................................................................................................................................. 09 Natural Area 52. West Anglian Plain ................................................................................................................................................ 14 Natural Area 54. Yardley-Whittlewood Ridge .............................................................................................................................. 20 Natural Area 55. Cotswolds ................................................................................................................................................................ 25 APPENDIX 3 Site of Special Scientifi c Interest Summaries .............................................................................................................................. 26 APPENDIX 4 Wildlife Site Summaries .................................................................................................................................................................... -

Our New Restaurant Now Openedtaste OFINDIAN NEPAL RESTAURANT All Day Indian Sizzler Bar Menu Available
15th July 2017 www.nenevalleynews.co.uk facebook.com/NeneValleyNews @NeneValleyNews Telephone: 01522 513515 We want to hear from you, so please submit your news story or event at NeneYour local independent community Valley www.nenevalleynews.co.uk newspaper for East Northamptonshire news everyday 5pm-7pmHAPPY HOUR Our new restaurant now openedTASTE OFINDIAN NEPAL RESTAURANT All day Indian Sizzler Bar Menu available. Call to book 01832 732320 • Open: 5:30-10pm, Monday to Saturday • The Bridge Hotel, Thrapston Knitting together Community In Brief to help under volunteers Official opening privileged create mosaic of Oundle Library children in Thrapston outdoor gym he “Friends of Inside... Page 4 Page 7 Oundle Library” Tare delighted to announce the opening of their new outdoor gym, the Police issue warning to schools formal opening of which takes place on Wednesday Detectives have written to every school in Northamptonshire warning to find someone’s exact location. It 19th July at 5.30pm. produces a map which allows you headteachers following online safety concerns about a live video streaming app. Northamptonshire to see their exact location down to Libraries are part of the the house they are in. Snapchat First for Wellbeing family, “hotspots” allow anyone to see delivering the Library taff from the POLIT the action to try to bring the Live.me live-streaming videos and watch snapchats that are set to public – Plus service on behalf of (Paedophile Online app to the attention of parents. others’ videos online, however the you do not need to have added any Northamptonshire County Investigation Team) have taken The app allows users to broadcast terms of use specify that users S of these people to get locations and Council. -
Sustainability Appraisal of the Core Spatial Strategy
Draft Sustainability Appraisal Scoping Report for the Four Towns Plan East Northamptonshire Local Plan November 2012 East Northamptonshire Local Plan: Draft Sustainability Appraisal Scoping Report for the Four Towns Plan (November 2012) Contents EXECUTIVE SUMMARY NON TECHNICAL SUMMARY 1 1. INTRODUCTION 3 BACKGROUND 3 THE FOUR TOWNS PLAN 4 WHAT IS SUSTAINABILITY APPRAISAL? 4 PURPOSE OF THE SCOPING REPORT 4 2. PLANS AND PROGRAMMES INFLUENCING THE FOUR 8 TOWNS PLAN AND SA (TASK A1) 3. BASELINE INFORMATION AND IDENTIFYING 8 SUSTAINABILITY ISSUES AND PROBLEMS (TASK A2 AND A3) Recent economic conditions 10 Settlement Pattern and Transport Links 13 The Economy 15 Retail and Leisure provision 18 Population 19 Deprivation 20 Housing 21 Health 23 Crime 24 Education 25 Biodiversity 26 Landscape 28 Cultural Heritage 28 Air 29 Water Quality and Resources 30 Soil and Land 31 Minerals and Waste 33 Energy and Climate Change 34 Green Infrastructure 36 4. SUSTAINABILITY FRAMEWORK 39 Development of Framework (Task A4) 39 Appraisal method 41 Monitoring 42 5. CONSULTATION ON SCOPE OF SUSTAINABILITY APPRAISAL 43 (TASK A5) Next Stages 44 APPENDIX 1: Review of Relevant Plans and Programmes APPENDIX 2: Census 2011 – summary information East Northamptonshire Local Plan: Draft Sustainability Appraisal Scoping Report for the Four Towns Plan (November 2012) Key website references: Northamptonshire Environmental Character and Green Infrastructure Study (River Nene Regional Park website): http://www.riverneneregionalpark.org/ Environment Agency Flood Maps: http://maps.environment- -

River Nene Waterspace Study
River Nene Waterspace Study Northampton to Peterborough RICHARD GLEN RGA ASSOCIATES November 2016 ‘All rights reserved. Copyright Richard Glen Associates 2016’ Richard Glen Associates have prepared this report in accordance with the instructions of their clients, Environment Agency & the Nenescape Landscape Partnership, for their sole DQGVSHFL¿FXVH$Q\RWKHUSHUVRQVZKRXVHDQ\LQIRUPDWLRQFRQWDLQHGKHUHLQGRVRDW their own risk. River Nene Waterspace Study River Nene Waterspace Study Northampton to Peterborough On behalf of November 2016 Prepared by RICHARD GLEN RGA ASSOCIATES River Nene Waterspace Study Contents 1.0 Introduction 3.0 Strategic Context 1.1 Partners to the Study 1 3.1 Local Planning 7 3.7 Vision for Biodiversity in the Nene Valley, The Wildlife Trusts 2006 11 1.2 Aims of the Waterspace Study 1 3.1.1 North Northamptonshire Joint Core Strategy 2011-2031 7 3.8 River Nene Integrated Catchment 1.3 Key Objectives of the Study 1 3.1.2 West Northamptonshire Management Plan. June 2014 12 1.4 Study Area 1 Joint Core Strategy 8 3.9 The Nene Valley Strategic Plan. 1.5 Methodology 2 3.1.3 Peterborough City Council Local Plan River Nene Regional Park, 2010 13 1.6 Background Research & Site Survey 2 Preliminary Draft January 2016 9 3.10 Destination Nene Valley Strategy, 2013 14 1.7 Consultation with River Users, 3.2 Peterborough Long Term Transport 3.11 A Better Place for All: River Nene Waterway Providers & Local Communities 2 Strategy 2011 - 2026 & Plan, Environment Agency 2006 14 Local Transport Plan 2016 - 2021 9 1.8 Report 2 3.12 Peterborough -
Appendix 2: Designated Sites Considered
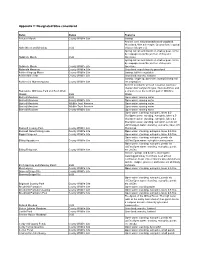
Appendix 2: Designated Sites considered Name Status Features Achurch Marsh County Wildlife Site Swamp Ancient semi-natural broadleaved woodland, Woodland, Wet ash-maple, Ground flora is typical Alder Wood and Meadow SSSI of base rich wet soil. Spring-fed fen and marsh on shallow peat, forme by seepage along the junction of clay and Aldwincle Marsh SSSI limestone. Spring-fed fen and marsh on shallow peat, forme by seepage along the junction of clay and Aldwincle Marsh County Wildlife Site limestone. Aldwincle Meadows County Wildlife Site Grassland: marsh/marshy grassland Ashton Kingcup Marsh County Wildlife Site Swamp: tall fen vegetation Ashton Mill Fields County Wildlife Site Grassland: marshy, lowland Swamp: single sp. dominant swamp/Swamp: tall Ashton Old Watermeadows County Wildlife Site fen vegetation Ancient woodland, wet ash Frazimus excelsio / mapler Acer campestre type. Narrow ditches and Badsaddle, Withmale Park and Bush Walk a stream cross the northern part of Wiltshire Woods SSSI Wood. Barford Meadows SSSI Open water: running water Barford Meadows County Wildlife Site Open water: running water Barford Meadows Wildlife Trust Reserve Open water: running water Barford Meadows Wildlife Trust Reserve Open water: running water Barford Meadows County Wildlife Site Open water: running water Open water: standing, eutrophic, lakes 0.5- 5ha/Open water: standing, eutrophic, lakes 0.5- 5ha/Open water: standing, eutrophic, lakes 0.5- 5ha/Open water: standing, eutrophic, ponds etc <0.5ha/Open water: standing, eutrophic, lakes 0.5- Barnwell Country Park County Wildlife Site 5ha/Scrub Barnwell Nene Fishing Lake County Wildlife Site Open water: standing, eutrophic, lakes 0.5-5ha Biggin Fishpond County Wildlife Site Open water: standing, eutrophic, lakes 0.5-5ha Open water: standing, eutrophic, ponds etc Billing Aquadrome County Wildlife Site <0.5ha/Open water: eutrophic running water Open water: standing, eutrophic, ponds etc <0.5ha/Open water: standing, eutrophic, ponds Billing Fishponds County Wildlife Site etc <0.5ha Stream, springs and flushes. -

A Fantastic Walk Including a Stately Home, Great 6.5 10.5 2 Hours Mainly Off Road
No Location Miles Km Time Difficulty Completed 1 Castle Ashby Circular: A fantastic walk including a stately home, great 6.5 10.5 2 hours Mainly off road. There are a few hills & especially a steep path up to Whiston church tea rooms, rivers, wildlife, spooky graveyards 2 Collingtree Circular: A golf course, open fields, villages, a canal, pocket 8 12.8 3.5 hours Mainly off road & along canal paths. Mostly flat easy walking, although there are several stiles to parks &, oh…an injury… negotiate 3 Braunston Circular: An ancient footpath, a gunpowder plot, a tale of 7.5 12 3 – 3.5 hours Mainly off road. There are quite a few hills and it can get muddy in winter, but it’s great to enjoy the woes, fab jams & marmalades & a canal finish changing seasons 4 Delapre Abbey Circular: A 10 mile walk from an ancient Abbey, along 10 16.1 3.5 hours A mix of off-road & on country lanes. As some tracks are around a lake & across fields it can get muddy the Nene way, through the washlands & beyond! in wet times 5 Brackley Circular: Market towns, fields, ‘Snakes (nearly) on a Plane’ & 6.5 10.5 2.5 hours Almost completely across fields (& aerodromes) frustration…”Get off my land!” 6 Great & Little Brington Circular: A wave to Diana.. 4.4 7.1 2 hours Mainly off road including across grass fields. There are a few steep up & down hills & it can get muddy in winter, but it’s great to enjoy the changing seasons 7 Yardley Gobian Circular: Stepping back in time across the fields to ‘The 3.7 5.95 90 mins This is a really undemanding walk which goes through two Northamptonshire villages. -

The Upper Nene Valley Gravel Pits Special Protection Area Supplementary Planning Document
The Upper Nene Valley Gravel Pits Special Protection Area Supplementary Planning Document Addendum to the SPA SPD: Mitigation Strategy Document for adoption approval Borough Council of Wellingborough – 31st October 2016 st East Northamptonshire Council – 21 November 2016 Please note that no change is being made to the already adopted SPA SPD, this addendum will slot into the end of the document as a new section 6. 1 CONTENTS Section Page 1 Purpose 4 2 Background 4 3 Regulations 5 4 Implications and Contribution to Access Management 5 5 Process 6 6 Payment mechanisms 8 7 Governance 8 8 Conclusions 8 Appendix 1 Map showing 3km zone around SPA 9 Appendix 2 Mitigation Needs Assessment 10 Appendix 3 Compliance with planning obligation tests 22 Appendix 4 S106 Wording 24 Appendix 5 Section 111 template 25 Definitions 26 This addendum (to the Special Protection Area SPD for the Upper Nene Valley Gravel Pits) applies to the council areas of East Northamptonshire and Wellingborough. These two authority areas in North Northamptonshire have the potential for residential sites to be located within the 3km buffer zone of the SPA, which is detailed further below. 2 1. Purpose 1.1 Local Planning Authorities have the duty as competent authorities under the Conservation of Habitats and Species Regulations 2010 (Habitat Regulations) to ensure that planning application decisions comply with the Habitats Regulations. The North Northamptonshire Joint Core Strategy 2011 – 2031 (Local Plan part 1) policy 4 safeguards the Upper Nene Valley Gravel Pits Special Protection Area, which was designated in April 2011 under the regulations, due to its number and type of bird species present. -

Download the Report Item 8 Upper
BOROUGH COUNCIL OF WELLINGBOROUGH AGENDA ITEM 8 Development Committee 31 October 2016 Report of Head of Planning and Local Development UPPER NENE VALLEY GRAVEL PITS SPECIAL PROTECTION AREA SUPPLEMENTARY PLANNING DOCUMENT (SPD) MITIGATION STRATEGY 1 Purpose of report To seek approval to adopt the mitigation strategy in Appendix 2, as an addendum to the Upper Nene Valley Gravel Pits Special Protection Area Supplementary Planning Document. 2 Executive summary A Supplementary Planning Document (SPD) was produced to help local planning authorities, developers and others ensure that development has no significant effect on the Upper Nene Valley Gravel Pits Special Protection Area (SPA), in accordance with the legal requirements of the Habitats Regulations. This was adopted at Services Committee on 14 September 2015. The Habitats Regulations Assessment (HRA) for the Joint Core Strategy identified that development across North Northamptonshire, specifically in East Northamptonshire and Wellingborough, is likely to have a significant effect on the Upper Nene Valley Gravel Pits Special Protection Area (SPA) and proposed a mitigation strategy to remove the significant effects. During the Joint Core Strategy (JCS) examination process Natural England, confirmed that this approach was necessary to ensure that the plan was legally compliant. A draft mitigation strategy has been the subject of consultation and this report seeks approval of an updated version taking account of comments received to be adopted as an addendum to the Upper Nene Valley Gravel Pits Special Protection Area SPD. 3. Appendices Appendix 1 – Consultation Statement including consultation comments and officer responses. Appendix 2 - Upper Nene Valley Gravel Pits Special Protection Area SPD mitigation strategy October 2016. -

Biodiversity Character Assessment Contents
BIODIVERSITY CHARACTER ASSESSMENT CONTENTS INTRODUCTION 5 Appointment and Brief 5 The Northamptonshire Environmental Characterisation Process 5 Characterisation in Practice 5 The Northamptonshire Biodiversity Character Assessment 5 Parallel Projects and Surveys 5 Structure of the Report 6 THE BIODIVERSITY RESOURCE 7 Introduction 7 Habitat and Species Loss 7 The Current Biodiversity Resource 7 THE BIODIVERSITY CHARACTER OF NORTHAMPTONSHIRE 15 Introduction 15 Biodiversity Character Type and Area Boundary Determination 15 1. Acid Sand 21 2. Liassic Slopes 32 3. Boulder Clay Woodlands 53 4. Cropped Clayland 70 5. Quarried Ironstone 91 6. Cropped Limestone Plateau 97 7. Limestone Woodlands 103 8. Limestone Slopes 111 9. Glacial Gravels 133 10. Minor Floodplain 136 11. Major Floodplain 162 GLOSSARY 173 BIBLIOGRAPHY 175 ACKNOWLEDGEMENTS 177 Henry Stanier - Clustered Bellflower BIODIVERSITY CHARACTER ASSESSMENT 2 1. INTRODUCTION 1.1 APPOINTMENT AND BRIEF In April 2003, as part of a Service Level Agreement, the Built and Natural Environment Section of Northamptonshire County Council appointed the Wildlife Trust for Northamptonshire to carry out a Biodiversity Character Assessment of Northamptonshire. However, due to staff changes at the Trust in July 2004, Denton Wood Associates was appointed to complete the project. 1.2 THE NORTHAMPTONSHIRE ENVIRONMENTAL CHARACTERISATION PROCESS The Biodiversity Character Assessment forms part of a wider project that seeks to deliver an integrated, robust and transparent Environmental Characterisation of Northamptonshire: the Northamptonshire Environmental Characterisation Process (ECP) through the integration of three parallel studies; the Historic, Biodiversity and Current Landscape Character Assessments, to produce the county’s Environmental Character Assessment (ECA). The principal objective of the overall project is to: • Develop key environmental baseline datasets that inform, develop and enhance the sustainable planning and management of the landscape. -

SLU3A NEWS Newsletter of the South Leicestershire U3A Website: Registered Charity Number 1122153 Issue No
SLU3A NEWS Newsletter of the South Leicestershire U3A Website: http://www.slu3a.org.uk/ Registered Charity Number 1122153 Issue No. 194 SEPTEMBER 2016 SEPTEMBER SONG/OPEN DAY/ALBERT HALL ‘It ‘s a long, long time, From May to September’ as the old song puts it. Hopefully the sun will con- tinue to shine, not least on our place in France where we will spend a couple of weeks, entertain- ing grandchildren, seeing old friends and going on our annual wine search. And if what ‘Brexit Means Brexit’ actually means is beginning to be- come a little clearer, one can’t help wondering what Brexit will mean for our stays in France. Will we be as unwelcome as some EU citizens currently feel here in the UK? After over twenty years we’ve made friends with many French people and re- gard our French place as much as home as our one OPEN DAY VISITORS –2015 in the UK. Ah well, not to worry—since we cannot possibly leave the EU before 2019, when your October sees our annual Open Day, when members editor will arrive at four score years, by that time have an opportunity to come and see what SLU3A he will probably be well into his dotage! has to offer from its many and varied groups. Come and see what the Quilters, Card Crafters, Needle Meanwhile, rumour has it (since we don’t have craft and Papercrafters get up to; Learn something the data) that many of our members actually don’t about Architecture, Transport or Science, how fran- take up the opportunities offered by our various tic is Exercise and Keep Fit or Tai Chi , or is 60s Music groups, trips, meetings to be active within the or- or Jazz your obsession? All these and many a more ganisation. -

Rivere Nene and Great Ouse Eutrophication Studies Final Report
60 ; * |( Rivere Nene and Great Ouse Eutrophication Studies Final Report En v ir o n m e n t A g e n c y NATIONAL LIBRARY & INFORMATION SERVICE ANGLIAN REGION Kingfisher House. Goldhay Way. Orton Goldhay. Peterborough PE2 5ZR August 1997 Michael Rose David Balbi ENVIRONMENT AGENCY 089682 Contents Page No. Sumnaiy Chapter Ode - Introduction 1 1.1 Introduction 1 1.2 Background to Project 2 1.3 Project Objectives 3 1.4 Phosphorus 4 1.5 Phytoplankton 4 1.6 Benthic Diatoms 6 1.7 Macrophyte 6 Chapter Two - Methods " 7 2.1 Sample Sites 7 2.2 Sampling Strategy 7 2.3 Phosphorus Data 10 2.4 Other Chemical Data 12 2.5 Biological Data 12 Chapter Three - River Great Ckse 16 3.1 Flow 16 3.2 Phosphorus in STW Effluent 16 3.3 Phosphorus in Rivers 18 3.4 Sources of Phosphorus 22 3.5 The Impact of P-Stripping on the River 24 3.6 Biological Data 28 Chapter Four - River Nene 37 4.1 Flow 37 4.2 Phosphorus in STW Effluents 38 4.3 Phosphorus in Rivers 39 4.4 Sources of Phosphorus 43 4.5 The Impact of P-Stripping on the River 46 4.6 Biological Data 50 Page No. Chapter Five - Discussion 60 5.1 Phosphorus at the STWs 60 5.2 Point Sources of Phosphorus 61 5.3 Other Factors Controlling Phosphorus Loads 62 5.4 Biological Responses 64 5.5 Hie River Phytoplankton Community 65 Chapter Six - Conclisions 69 Chapter Seven - Recommendations 70 7.1 Research 70 7.2 Monitoring 70 7.3 Monitoring Programme Guidelines 70 References 72 Acknowledgments 77 4 Appendices \ list of Tables Page No.