The Effects of a Changing Climate on Key Habitats in Alaska
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

North Pacific Research Board Project Final Report
NORTH PACIFIC RESEARCH BOARD PROJECT FINAL REPORT Synthesis of Marine Biology and Oceanography of Southeast Alaska NPRB Project 406 Final Report Ginny L. Eckert1, Tom Weingartner2, Lisa Eisner3, Jan Straley4, Gordon Kruse5, and John Piatt6 1 Biology Program, University of Alaska Southeast, and School of Fisheries and Ocean Sciences, University of Alaska Fairbanks, 11120 Glacier Hwy., Juneau, AK 99801, (907) 796-6450, [email protected] 2 Institute of Marine Science, University of Alaska Fairbanks, P.O. Box 757220, Fairbanks, AK 99775-7220, (907) 474-7993, [email protected] 3 Auke Bay Lab, National Oceanic and Atmospheric Administration, 17109 Pt. Lena Loop Rd., Juneau, AK 99801, (907) 789-6602, [email protected] 4 University of Alaska Southeast, 1332 Seward Ave., Sitka, AK 99835, (907) 774-7779, [email protected] 5 School of Fisheries and Ocean Sciences, University of Alaska Fairbanks, 11120 Glacier Hwy., Juneau, AK 99801, (907) 796-2052, [email protected] 6 Alaska Science Center, US Geological Survey, Anchorage, AK, 360-774-0516, [email protected] August 2007 ABSTRACT This project directly responds to NPRB specific project needs, “Bring Southeast Alaska scientific background up to the status of other Alaskan waters by completing a synthesis of biological and oceanographic information”. This project successfully convened a workshop on March 30-31, 2005 at the University of Alaska Southeast to bring together representatives from different marine science disciplines and organizations to synthesize information on the marine biology and oceanography of Southeast Alaska. Thirty-eight individuals participated, including representatives of the University of Alaska and state and national agencies. -

Pacific Ocean: Supplementary Materials
CHAPTER S10 Pacific Ocean: Supplementary Materials FIGURE S10.1 Pacific Ocean: mean surface geostrophic circulation with the current systems described in this text. Mean surface height (cm) relative to a zero global mean height, based on surface drifters, satellite altimetry, and hydrographic data. (NGCUC ¼ New Guinea Coastal Undercurrent and SECC ¼ South Equatorial Countercurrent). Data from Niiler, Maximenko, and McWilliams (2003). 1 2 S10. PACIFIC OCEAN: SUPPLEMENTARY MATERIALS À FIGURE S10.2 Annual mean winds. (a) Wind stress (N/m2) (vectors) and wind-stress curl (Â10 7 N/m3) (color), multiplied by À1 in the Southern Hemisphere. (b) Sverdrup transport (Sv), where blue is clockwise and yellow-red is counterclockwise circulation. Data from NCEP reanalysis (Kalnay et al.,1996). S10. PACIFIC OCEAN: SUPPLEMENTARY MATERIALS 3 (a) STFZ SAFZ PF 0 100 5.5 17 200 18 16 6 5 4 9 4.5 13 12 15 14 Potential 300 11 10 temperature Depth (m) 6.5 400 9 (°C) 8 7 3.5 500 8 Subtropical Domain Transition Zone Subarctic Domain Alaskan STFZ SAFZ Stream (b) 0 35.2 34.6 34 33 32.7 32.8 100 33.7 33.8 200 34.5 34.3 300 34 34.2 33.9 Depth (m) 34.1 400 34 34.1 Salinity 500 (c) 30°N 40°N 50°N 0 100 2 1 4 8 6 200 10 20 12 14 16 44 25 44 30 300 12 14 35 Depth (m) 16 400 20 40 Nitrate (μmol/kg) 500 (d) 30°NLatitude 40°N 50°N 24.0 Sea surface density Nitrate (μmol/kg) 24.5 θ σ 25.0 1 2 25.5 1 10 2 4 12 8 26.0 14 Potential density 10 12 16 16 26.5 20 25 30 40 35 27.0 30°N 40°N 50°N FIGURE S10.3 The subtropical-subarctic transition along 150 W in the central North Pacific (MayeJune, 1984). -

Climate Change in Alaska
CLIMATE CHANGE ANTICIPATED EFFECTS ON ECOSYSTEM SERVICES AND POTENTIAL ACTIONS BY THE ALASKA REGION, U.S. FOREST SERVICE Climate Change Assessment for Alaska Region 2010 This report should be referenced as: Haufler, J.B., C.A. Mehl, and S. Yeats. 2010. Climate change: anticipated effects on ecosystem services and potential actions by the Alaska Region, U.S. Forest Service. Ecosystem Management Research Institute, Seeley Lake, Montana, USA. Cover photos credit: Scott Yeats i Climate Change Assessment for Alaska Region 2010 Table of Contents 1.0 Introduction ........................................................................................................................................ 1 2.0 Regional Overview- Alaska Region ..................................................................................................... 2 3.0 Ecosystem Services of the Southcentral and Southeast Landscapes ................................................. 5 4.0 Climate Change Threats to Ecosystem Services in Southern Coastal Alaska ..................................... 6 . Observed changes in Alaska’s climate ................................................................................................ 6 . Predicted changes in Alaska climate .................................................................................................. 7 5.0 Impacts of Climate Change on Ecosystem Services ............................................................................ 9 . Changing sea levels ............................................................................................................................ -

Climate Change in Kiana, Alaska Strategies for Community Health
Climate Change in Kiana, Alaska Strategies for Community Health ANTHC Center for Climate and Health Funded by Report prepared by: Michael Brubaker, MS Raj Chavan, PE, PhD ANTHC recognizes all of our technical advisors for this report. Thank you for your support: Gloria Shellabarger, Kiana Tribal Council Mike Black, ANTHC Linda Stotts, Kiana Tribal Council Brad Blackstone, ANTHC Dale Stotts, Kiana Tribal Council Jay Butler, ANTHC Sharon Dundas, City of Kiana Eric Hanssen, ANTHC Crystal Johnson, City of Kiana Oxcenia O’Domin, ANTHC Brad Reich, City of Kiana Desirae Roehl, ANTHC John Chase, Northwest Arctic Borough Jeff Smith, ANTHC Paul Eaton, Maniilaq Association Mark Spafford, ANTHC Millie Hawley, Maniilaq Association Moses Tcheripanoff, ANTHC Jackie Hill, Maniilaq Association John Warren, ANTHC John Monville, Maniilaq Association Steve Weaver, ANTHC James Berner, ANTHC © Alaska Native Tribal Health Consortium (ANTHC), October 2011. Funded by United States Indian Health Service Cooperative Agreement No. AN 08-X59 Through adaptation, negative health effects can be prevented. Cover Art: Whale Bone Mask by Larry Adams TABLE OF CONTENTS Summary 1 Introduction 5 Community 7 Climate 9 Air 13 Land 15 Rivers 17 Water 19 Food 23 Conclusion 27 Figures 1. Map of Maniilaq Service Area 6 2. Google Maps view of Kiana and region 8 3. Mean Monthly Temperature Kiana 10 4. Mean Monthly Precipitation Kiana 11 5. Climate Change Health Assessment Findings, Kiana Alaska 28 Appendices A. Kiana Participants/Project Collaborators 29 B. Kiana Climate and Health Web Resources 30 C. General Climate Change Adaptation Guidelines 31 References 32 Rural Arctic communities are vulnerable to climate change and residents seek adaptive strategies that will protect health and health infrastructure. -

INTERANNUAL VARIATIONS in ZOOPLANKTON BIOMASS in the GULF of ALASKA, and COVARIATION with CALIFORNIA CURRENT ZOOPLANKTON BIOMASS Iiichaiw I)
BRODEUR ET AL.: VARIABILITY IN ZOOPLANKTON BIOMASS CalCOFl Rep., Vol. 37, 1996 INTERANNUAL VARIATIONS IN ZOOPLANKTON BIOMASS IN THE GULF OF ALASKA, AND COVARIATION WITH CALIFORNIA CURRENT ZOOPLANKTON BIOMASS IiICHAIW I). Ul1OIlEUK UliUCE W. FllOST STEVEN R.HAKE Alaska Fihrirs Science Criicrr, NOAA Sc.hool of Ocranograpliy Intcriintioii~l1'~ciiic tfhbiit <:omtiiii\ion 7600 Sand Point Wq NE 1301 357WJ V.0. Box 95009 Sr'lttle, W.lshlllgton '181 15 Uiii\ cr\ii). of W.i\hingtmi ScJttlr, W'lstlltl~t~~n981 45 Se'lttlr. Washln~on981 95 ABSTRACT tinguish their relative contributions (Wickett 1967; Large-scale atmospheric and oceanographic condi- Chelton et al. 1982; Roessler aiid Chelton 1987). tions affect the productivity of oceanic ecosystems both It has become increasingly apparent that atmospheric locally and at some distance froin the forcing mecha- and oceanic conditions are likely to change due to a nism. Recent studies have suggested that both the buildup of greenhouse gases in the atmosphere (Graham Subarctic Domain of the North Pacific Ocean and the 1995). Although there has been much interest in pre- California Current have undergone dramatic changes in dicting the effects of climate change, especially on fish- zooplankton biomass that appear to be inversely related eries resources (e.g., see papers in Beaniish 1995), dif- to each other. Using time series and correlation analy- ferent scenarios exist for future trends in basic physical ses, we characterized the historical nature of zooplank- processes such as upwelling (Bakun 1990; Hsieh and ton biomass at Ocean Station P (50"N,145"W) and froiii Boer 1992). Biological processes are niore laborious to offshore stations in the CalCOFI region. -
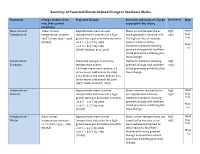
Summary of Expected Climate-‐Related Change in Southeast Alaska
Summary of Expected Climate-Related Change in Southeast Alaska Parameter Change to date (if no Projected Change Direction and range of change Confidence Map info, then current expected in the future condition) Mean Annual Mean annual Approximate mean annual Mean annual temperatures High SNAP Temperature temperature increase: temperature increases for a high are expected to increase with >95% Map +0.8°C from 1943 – 2005 greenhouse gas emissions scenario: the highest rate of increase Tool (NOAA) +0.5°C – 3.5°C by 2050 seen in winter months. Maps +2.0°C – 6.0°C by 2100 Northern mainland showing 1-3 (SNAP; Wolken et al. 2011) greatest change and southern island provinces exhibiting the least change. Temperature – Projected changes in extreme Northern mainland showing High Extremes temperature events: greatest change and southern >95% 3-6 times more warm events; 3-5 island provinces exhibiting the times fewer cold events by 2050. least change. 5-8.5 times more warm events; 8-12 times fewer cold events by 2100. (NPS; Timlin & Walsh, 2007) Temperature – Approximate mean summer Mean summer temperatures High SNAP Summer temperature increases for a high are expected to increase. >95% Map greenhouse gas emissions scenario: Northern mainland showing Tool +0.5°C – 2.0°C by 2050 greatest change and southern Maps +2.0°C – 5.5°C by 2100 island provinces exhibiting the 4-6 (SNAP) least change. Temperature – Mean winter Approximate mean winter Mean winter temperatures are High SNAP Winter temperature increase: temperature increases for a high expected to increase at an >95% Map +1.1°C from 1943 – 2005 greenhouse gas emissions scenario: elevated level compared to Tool (NOAA) +1.0°C – 3.5°C by 2050 other seasons. -

Downloaded 09/28/21 07:00 PM UTC
AUGUST 2005 C A P OTONDI ET AL. 1403 Low-Frequency Pycnocline Variability in the Northeast Pacific ANTONIETTA CAPOTONDI AND MICHAEL A. ALEXANDER NOAA/CIRES Climate Diagnostics Center, Boulder, Colorado CLARA DESER National Center for Atmospheric Research,* Boulder, Colorado ARTHUR J. MILLER Scripps Institution of Oceanography, La Jolla, California (Manuscript received 14 January 2004, in final form 23 November 2004) ABSTRACT The output from an ocean general circulation model (OGCM) driven by observed surface forcing is used in conjunction with simpler dynamical models to examine the physical mechanisms responsible for inter- annual to interdecadal pycnocline variability in the northeast Pacific Ocean during 1958–97, a period that includes the 1976–77 climate shift. After 1977 the pycnocline deepened in a broad band along the coast and shoaled in the central part of the Gulf of Alaska. The changes in pycnocline depth diagnosed from the model are in agreement with the pycnocline depth changes observed at two ocean stations in different areas of the Gulf of Alaska. A simple Ekman pumping model with linear damping explains a large fraction of pycnocline variability in the OGCM. The fit of the simple model to the OGCM is maximized in the central part of the Gulf of Alaska, where the pycnocline variability produced by the simple model can account for ϳ70%–90% of the pycnocline depth variance in the OGCM. Evidence of westward-propagating Rossby waves is found in the OGCM, but they are not the dominant signal. On the contrary, large-scale pycnocline depth anomalies have primarily a standing character, thus explaining the success of the local Ekman pumping model. -

The Alaskan Stream
1 THE ALASKAN STREAM by Felix Favorite Biological Laboratory, Bureau of Commercial Fisheries Seattle, Washington, June 1965 ABSTRACT Relative currents . 11 The general oceanographic features and continuity of the Dynamic topography, 0/300 m . II Alaskan Stream are discussed using data obtained during May Dynamic topography, 300/1,000 m. ........... .. ... 12 through August 1959. The Alaskan Stream is defined as the Dynamic topography, 0/1,000 m . ... .. ... ... .... 12 extension of the Alaska Current which flows westward along the TRANSPORT • • • • • • • • • • • • • • • • • • • • • . • • • • • • • • • • • • • • • • • • • 12 south side of the Aleutian Islands. It is continuous as far west Relative transport . • . 12 ward as long. I 70°E where it divides sending one branch north Transport, 0 to 1,000 m . 14 ward into the Bering Sea and one southwestward to rejoin the Wind-driven transport . 14 eastward flowing Subarctic Current. Sea level pressure and wind stress................... 15 Observed westward velocities near Atka and Adak Islands were Ekman transport .. 17 in excess of 100 em/sec, but maximum geostrophic velocities Total transport . 17 (referred to 1000-m level) of only 30 em/sec were obtained from Comparison of theoretical and relative transports. 18 station data. Volume transport, computed from geostrophic CoNCLUSIONS........ ... .. .. .... .. .............. 18 currents, was approximately 6 X 108 m8/sec. LITERATURE CITED •• • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • 19 Evidence is presented that the Alaskan Stream is driven pri marily by the action of wind stress. The observed narrowness of ACKNOWLEDGMENTS the stream and continuity of transport also support the view that I am indebted to Dr. W. B. McAlister, Bureau of it is a western boundary current related to the general distribution of wind stress. -

Gulf of Alaska Ocean and Climate Changes
Harbo Rick © Gulf of Alaska Changes Climate and Ocean [153] 86587_p153_176.indd 153 12/30/04 4:42:57 PM highlights ■ For decades, the depth of the surface mixed layer sharks, skates, several forage fi sh species, and had become increasingly shallow, reducing nutrient yellow Irish lord have signifi cantly increased in levels. A deepening of the mixed layer from 1999- abundance and/or frequency of occurrence since 2002 temporarily reduced this trend, but the winter 1990. of 2002/03 was the shallowest on record. abundance and recruitment of many salmon stocks ■ Phytoplankton blooms on the shelf were stronger in was above average for much of the 1980/90s, 1999 and 2000 than 1998 and 2001. while catches have decreased slightly over the past decade. Recruitment of all brood years ■ The seasonal peak of Neocalanus copepods at Ocean through at least the mid-1990s was strong. Station P was early in the 1980/90s and returned to the long-term average from 1999 – 2001. in spite of moderate declines in abundance and Ocean below-average recruitment of some commercial ■ After 1976/77, the groundfi sh community had stocks, most groundfi sh, salmon, and herring relatively stable species composition and abundance and fi sheries remained healthy throughout 2002 with of individual species but, several signifi cant changes no indications of overfi shing or “fi shing down the have occurred: Climate food web.” a general decline in walleye pollock biomass over king crab and shrimp stocks have not recovered the past decade, but with strong recruitment from a collapse in the early 1980s. -

Cook Inlet Circulation Model Calculations Final Report to the U.S
OCS Study BOEM 2015-050 Cook Inlet Circulation Model Calculations Final Report to the U.S. Department of the Interior Bureau of Ocean Energy Management Contract No. M14AC00014 Seth L. Danielson1 [email protected] Katherine S. Hedstrom1 [email protected] 2 Enrique Curchitser [email protected] 1Institute of Marine Science 2Institute of Marine and Coastal Sciences School of Fisheries and Ocean Science Rutgers University University of Alaska Fairbanks 71 Dudley Rd., New Brunswick, NJ 08901 905 N. Koyukuk Dr., Fairbanks AK, 99775 732-932-7889 (Office) 907 474-7834 (Office) 732- 932-8578 (Fax) 907 474 7204 (Fax) OCS Study BOEM 2015-050 Cook Inlet Circulation Model Calculations This collaboration between the U.S. Department of the Interior Bureau of Ocean Energy Management, the University of Alaska Fairbanks and Rutgers University was funded by the the U.S. Department of the Interior Bureau of Ocean Energy Management Alaska Outer Continental Shelf Region Anchorage AK 99503 under Contract No. M14AC00014 as part of the Bureau of Ocean Energy Management Alaska Environmental Studies Program 18 November 2016 Citation: Danielson, S. L., K. S. Hedstrom, E. Curchitser, 2016. Cook Inlet Model Calculations, Final Report to Bureau of Ocean Energy Management, M14AC00014, OCS Study BOEM 2015- 050, University of Alaska Fairbanks, Fairbanks, AK, 149 pp. Cover Image: NWGOA model sea surface salinity and Aqua Satellite 250 m pixel resolution false- color image of the northern Gulf of Alaska. Aqua image provided by NASA Rapid Response Land, Atmosphere Near real-time Capability for EOS (LANCE) system operated by the NASA/GSFC/Earth Science Data and Information System (ESDIS) with funding provided by NASA/HQ. -

Assessment of the Potential Health Impacts of Climate Change in Alaska
Department of Health and Social Services Division of Public Health Editors Valerie J. Davidson, Commissioner Jay C. Butler, MD, Chief Medical Officer Joe McLaughlin, MD, MPH and Director Louisa Castrodale, DVM, MPH 3601 C Street, Suite 540 Local (907) 269-8000 Anchorage, Alaska 99503 http://dhss.alaska.gov/dph/Epi 24 Hour Emergency 1-800-478-0084 Volume 20 Number 1 Assessment of the Potential Health Impacts of Climate Change in Alaska Contributed by Sarah Yoder, MS, Alaska Section of Epidemiology January 8, 2018 Acknowledgments: We thank the following people for their contributions to this report: Dr. Sandrine Deglin and Madison Pachoe, Alaska Section of Epidemiology; Dr. Bob Gerlach, Alaska Department of Environmental Conservation; Dr. James Fall, Alaska Department of Fish and Game; Michael Brubaker, Alaska Native Tribal Health Consortium; Dr. Robin Bronen, Alaska Institute for Justice; Dr. Tom Hennessey, CDC Arctic Investigations Program; and Dr. Ali Hamade, Dr. Paul Anderson, and Dr. Yuri Springer, former Alaska Section of Epidemiology staff. I TABLE OF CONTENTS EXECUTIVE SUMMARY .......................................................................................................................... V Key Potential Adverse Health Impacts by Health Effect Category .............................................. VII Summary Recommendations ........................................................................................................... X 1.0 INTRODUCTION AND OVERVIEW ............................................................................................1 -

Ocean Current
Ocean current Ocean current is the general horizontal movement of a body of ocean water, generated by various factors, such as earth's rotation, wind, temperature, salinity, tides etc. These movements are occurring on permanent, semi- permanent or seasonal basis. Knowledge of ocean currents is essential in reducing costs of shipping, as efficient use of ocean current reduces fuel costs. Ocean currents are also important for marine lives, as well as these are required for maritime study. Ocean currents are measured in Sverdrup with the symbol Sv, where 1 Sv is equivalent to a volume flow rate of 106 cubic meters per second (0.001 km³/s, or about 264 million U.S. gallons per second). On the other hand, current direction is called set and speed is called drift. Causes of ocean current are a complex method and not yet fully understood. Many factors are involved and in most cases more than one factor is contributing to form any particular current. Among the many factors, main generating factors of ocean current are wind force and gradient force. Current caused by wind force: Wind has a tendency to drag the uppermost layer of ocean water in the direction, towards it is blowing. As well as wind piles up the ocean water in the wind blowing direction, which also causes to move the ocean. Lower layers of water also move due to friction with upper layer, though with increasing depth, the speed of the wind-induced current becomes progressively less. As soon as any motion is started, then the Coriolis force (effect of earth’s rotation) also starts working and this Coriolis force causes the water to move to the right in the northern hemisphere and to the left in the southern hemisphere.