Mating Success 15
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Check List of Gerromorpha (Hemiptera) from India
Rec. zool. Surv. India: 100 (Part 1-2) : 55-97, 2002 A CHECK LIST OF GERROMORPHA (HEMIPTERA) FROM INDIA G. THIRUMALAI Zoological Survey of India, Southern Regional Station, Chennai 600 028. INTRODUCflON The Infraorder Gerromorpha comprises of semi-aquatic bugs characterised by long conspicuous antennae, longer than head and inserted in front of eyes. They are distributed in all kinds of climatic zones, except the coldest and driest parts. This infraorder contains 8 families namely, Gerridae, Veliidae, Hydrometridae, Mesoveliidae, Hebridae, Macroveliidae, Paraphrynoveliidae and Hennatobatidae (Andersen, 1982a). Knowledge of Indian semi-aquatic Hemiptera is limited to the taxonomic preliminaries, recording species from different parts of the country. (Distant, 1903a; 1910 a&b; Annandale, 1919; Bergroth, 1915a; Paiva, 1919a & b; Dover, 1928; Hafiz & Mathai, 1938; Hafiz & Riberio, 1939; Hafiz & Pradhan, 1947; Pradhan, 1950a, b& 1975; Gupta, 1981; Selvanayagam, 1981; Roy et al. 1988; Ghosh et al. 1989; Polhenlus & Starmuhlner, 1990; Bal & Basu, 1994 & 1997; Chen & Zettel, 1999). The revisionary work of Andersen (1975, 1980, 1990 & 1993); Den Boer (1969); Hungerford & Matsuda (1958a & b, 1960, 1962b & 1965); Herring (1961); Polhemus & Andersen (1984); Andersen & Foster (1992); Andersen & Chen (1993); Chen & Nieser (1993a & b); Polhemus & Karunaratne (1993); Polhemus (1994); Polhemus & Polhemus (1994 & 1995a) on a few genera of Gerridae; Lundblad (1936) on the genera Rhagovelia Mayr and Tetraripis Lundblad; Andersen (1981 b, 1983 & 1989); Polhemus -

Metacommunities and Biodiversity Patterns in Mediterranean Temporary Ponds: the Role of Pond Size, Network Connectivity and Dispersal Mode
METACOMMUNITIES AND BIODIVERSITY PATTERNS IN MEDITERRANEAN TEMPORARY PONDS: THE ROLE OF POND SIZE, NETWORK CONNECTIVITY AND DISPERSAL MODE Irene Tornero Pinilla Per citar o enllaçar aquest document: Para citar o enlazar este documento: Use this url to cite or link to this publication: http://www.tdx.cat/handle/10803/670096 http://creativecommons.org/licenses/by-nc/4.0/deed.ca Aquesta obra està subjecta a una llicència Creative Commons Reconeixement- NoComercial Esta obra está bajo una licencia Creative Commons Reconocimiento-NoComercial This work is licensed under a Creative Commons Attribution-NonCommercial licence DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode Irene Tornero Pinilla 2020 DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode IRENE TORNERO PINILLA 2020 DOCTORAL PROGRAMME IN WATER SCIENCE AND TECHNOLOGY SUPERVISED BY DR DANI BOIX MASAFRET DR STÉPHANIE GASCÓN GARCIA Thesis submitted in fulfilment of the requirements to obtain the Degree of Doctor at the University of Girona Dr Dani Boix Masafret and Dr Stéphanie Gascón Garcia, from the University of Girona, DECLARE: That the thesis entitled Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode submitted by Irene Tornero Pinilla to obtain a doctoral degree has been completed under our supervision. In witness thereof, we hereby sign this document. Dr Dani Boix Masafret Dr Stéphanie Gascón Garcia Girona, 22nd November 2019 A mi familia Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. -

Distribution of Spiders in Coastal Grey Dunes
kaft_def 7/8/04 11:22 AM Pagina 1 SPATIAL PATTERNS AND EVOLUTIONARY D ISTRIBUTION OF SPIDERS IN COASTAL GREY DUNES Distribution of spiders in coastal grey dunes SPATIAL PATTERNS AND EVOLUTIONARY- ECOLOGICAL IMPORTANCE OF DISPERSAL - ECOLOGICAL IMPORTANCE OF DISPERSAL Dries Bonte Dispersal is crucial in structuring species distribution, population structure and species ranges at large geographical scales or within local patchily distributed populations. The knowledge of dispersal evolution, motivation, its effect on metapopulation dynamics and species distribution at multiple scales is poorly understood and many questions remain unsolved or require empirical verification. In this thesis we contribute to the knowledge of dispersal, by studying both ecological and evolutionary aspects of spider dispersal in fragmented grey dunes. Studies were performed at the individual, population and assemblage level and indicate that behavioural traits narrowly linked to dispersal, con- siderably show [adaptive] variation in function of habitat quality and geometry. Dispersal also determines spider distribution patterns and metapopulation dynamics. Consequently, our results stress the need to integrate knowledge on behavioural ecology within the study of ecological landscapes. / Promotor: Prof. Dr. Eckhart Kuijken [Ghent University & Institute of Nature Dries Bonte Conservation] Co-promotor: Prf. Dr. Jean-Pierre Maelfait [Ghent University & Institute of Nature Conservation] and Prof. Dr. Luc lens [Ghent University] Date of public defence: 6 February 2004 [Ghent University] Universiteit Gent Faculteit Wetenschappen Academiejaar 2003-2004 Distribution of spiders in coastal grey dunes: spatial patterns and evolutionary-ecological importance of dispersal Verspreiding van spinnen in grijze kustduinen: ruimtelijke patronen en evolutionair-ecologisch belang van dispersie door Dries Bonte Thesis submitted in fulfilment of the requirements for the degree of Doctor [Ph.D.] in Sciences Proefschrift voorgedragen tot het bekomen van de graad van Doctor in de Wetenschappen Promotor: Prof. -

Heteroptera: Gerromorpha) in Central Europe
Shortened web version University of South Bohemia in České Budějovice Faculty of Science Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe RNDr. Tomáš Ditrich Ph.D. Thesis Supervisor: Prof. RNDr. Miroslav Papáček, CSc. University of South Bohemia, Faculty of Education České Budějovice 2010 Shortened web version Ditrich, T., 2010: Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe. Ph.D. Thesis, in English. – 85 p., Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic. Annotation Ecology of Veliidae and Mesoveliidae (Hemiptera: Heteroptera: Gerromorpha) was studied in selected European species. The research of these non-gerrid semiaquatic bugs was especially focused on voltinism, overwintering with physiological consequences and wing polymorphism with dispersal pattern. Hypotheses based on data from field surveys were tested by laboratory, mesocosm and field experiments. New data on life history traits and their ecophysiological consequences are discussed in seven original research papers (four papers published in peer-reviewed journals, one paper accepted to publication, one submitted paper and one communication in a conference proceedings), creating core of this thesis. Keywords Insects, semiaquatic bugs, life history, overwintering, voltinism, dispersion, wing polymorphism. Financial support This thesis was mainly supported by grant of The Ministry of Education, Youth and Sports of the Czech Republic No. MSM 6007665801, partially by grant of the Grant Agency of the University of South Bohemia No. GAJU 6/2007/P-PřF, by The Research Council of Norway: The YGGDRASIL mobility program No. 195759/V11 and by Czech Science Foundation grant No. 206/07/0269. Shortened web version Declaration I hereby declare that I worked out this Ph.D. -

(AMMODRAMUS NELSONI) SPARROWS by Jennifer Walsh University of New Hampshire, September 2015
HYBRID ZONE DYNAMICS BETWEEN SALTMARSH (AMMODRAMUS CAUDACUTUS) AND NELSON’S (AMMODRAMUS NELSONI) SPARROWS BY JENNIFER WALSH Baccalaureate Degree (BS), University of New Hampshire, 2007 Master’s Degree (MS), University of New Hampshire, 2009 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Natural Resources and Environmental Studies September, 2015 This dissertation has been examined and approved in partial fulfillment of the requirements for the degree of Natural Resources and Environmental Studies in the Department of Natural Resources and Earth Systems Science by: Dissertation Director, Adrienne I. Kovach Research Associate Professor of Natural Resources Brian J. Olsen, Assistant Professor, School of Biology and Ecology, University of Maine W. Gregory Shriver, Associate Professor of Wildlife Ecology, University of Delaware Rebecca J. Rowe, Assistant Professor of Natural Resources Kimberly J. Babbitt, Professor of Natural Resources On July 9, 2015 Original approval signatures are on file with the University of New Hampshire Graduate School. ACKNOWLEDGEMENTS While pursuing my doctorate, a number of people have helped me along the way, and to each of them I am exceedingly grateful. First and foremost, I am grateful for my advisor, Adrienne Kovach, for her guidance, support, and mentorship. Without her, I would not be where I am, and I will be forever grateful for the invaluable role she has played in my academic development. Her unwavering enthusiasm for the project and dedication to its success has been a source of inspiration and I will never forget our countless road trips and long days out on the marsh. -

Systematics, Historical Biogeography and Ecological Phylogenetics in A
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Damgaard Jakob Artikel/Article: Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders 813-822 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Systematics, Historical Biogeography and Ecological Phylogenetics in a clade of water striders1 J. DAMGAARD Abstract: I hereby review the current knowledge about systematics, historical biogeography and ecolo- gical phylogenetics in the three principal northern temperate genera of water striders Limnoporus STÅL 1868, Aquarius SCHELLENBERG 1800 and Gerris FABRICIUS 1794. Most of the discussion is based on com- parison of a recently published combined analysis tree involving four genetic markers and a morpholo- gical data set with older phylogenetic trees primarily based on manual cladistic optimization of mor- phological characters. Key words: DNA-barcodes, Gerrinae, phylogeography, simultaneous analyses. Introduction nally, water striders show great variation in mating strategies, and morphological and Water striders (Hemiptera-Heteroptera, behavioral adaptations to accomplish or Gerromorpha, Gerridae) are familiar inhab- avoid multiple mating (ANDERSEN 1994, itants of aquatic habitats throughout the 1996; ARNQVIST 1997). The striking diver- Worlds temperate, subtropical, and tropical sity in habitat selection, wing polymorphism regions comprising approximately 640 de- and mating strategies – along with the prac- scribed species in 72 genera (ANDERSEN & tically two dimensional habitat, has made WEIR 2004). Most water striders are found water striders popular objects in studies of in freshwater habitats, such as rivers, behavior, ecology and evolution (SPENCE & streams, lakes and ponds, but a few genera ANDERSEN 1994; ROWE et al. -
Water Bug ID Guide
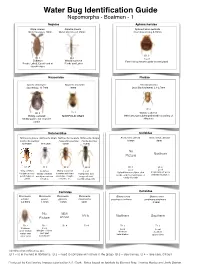
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Insecta: Heteroptera)
340 _____________Mun. Ent. Zool. Vol. 4, No. 2, June 2009__________ GERROMORPHAN FAUNA OF ÇORUM PROVINCE IN TURKEY (INSECTA: HETEROPTERA) Ali Salur* and Seda Mesci** * Hitit University, Faculty of Science and Arts, Department of Biology, 19030 Çorum – Turkey. E-mail: [email protected] ** Gazi University, Institute of Science, Department of Biology, 06500 Ankara – Turkey. E- mail: [email protected] [Salur, A. & Mesci, S. 2009. Gerromorphan fauna of Çorum province in Turkey (Insecta: Heteroptera). Munis Entomology & Zoology 4 (2): 340-345] ABSTRACT: As a result of revised literatures for determination of the Gerromorphan fauna of Çorum province and a scientific excursion in Çorum province between the years 2006- 2008, 9 Gerromorphan species were found. All species are new records to Çorum province and the Black Sea Region of Turkey. KEY WORDS: Heteroptera, Gerromorpha, Gerridae, Hydrometridae, Veliidae, Çorum, Turkey. Çorum province is in the Black Sea Region of Turkey (Fig. 1). The research area is located in 39°53'-, 41°20'N North latitute and 33°57' - 35°33' East longitute and the altitude of Çorum is between about 800 m-1600 m. As a result of the researching reviewed literatures on Turkish Gerromorphan insects, it was determined that any taxa are known in Çorum. The aim of this study is to determine the Gerromorphan fauna of Çorum. 384 specimens were collected with field guides between April-September in 2006-2008 in Çorum located in Black Sea Region of Turkey. 9 species belong to 4 genus of 3 families in infraorder Gerromorphan were determined. One species of Veliidae, one species of Hydrometridae and seven species of Gerridae were collected from 15 localities in various aquatic habitats of the Çorum province. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Morphology Reveals the Unexpected Cryptic Diversity in Ceratophyllus Gallinae (Schrank, 1803) Infested Cyanistes Caeruleus Linnaeus, 1758 Nest Boxes
Acta Parasitologica (2020) 65:874–881 https://doi.org/10.1007/s11686-020-00239-6 ORIGINAL PAPER Morphology Reveals the Unexpected Cryptic Diversity in Ceratophyllus gallinae (Schrank, 1803) Infested Cyanistes caeruleus Linnaeus, 1758 Nest Boxes Olga Pawełczyk1 · Tomasz Postawa2 · Marian Blaski3 · Krzysztof Solarz1 Received: 6 June 2019 / Accepted: 29 May 2020 / Published online: 8 June 2020 © The Author(s) 2020 Abstract Purpose The main aim of our study was to examine morphological diferentiation between and within sex of hen feas— Ceratophyllus gallinae (Schrank, 1803) population collected from Eurasian blue tit (Cyanistes caeruleus Linnaeus, 1758), inhabiting nest boxes and to determine the morphological parameters diferentiating this population. Methods A total of 296 feas were collected (148 females and 148 males), determined to species and sex, then the following characters were measured in each of the examined feas: body length, body width, length of head, width of head, length of comb, height of comb, length of tarsus, length of thorax and length of abdomen. Results The comparison of body size showed the presence of two groups among female and male life forms of the hen fea, which mostly difered in length of abdomen, whereas the length of head and tarsus III were less variable. Conclusion Till now, the only certain information is the presence of two adult life forms of C. gallinae. The genesis of their creation is still unknown and we are not able to identify the mechanism responsible for the morphological diferentiation of feas collected from the same host. In order to fnd answer to this question, future research in the feld of molecular taxonomy is required. -

A Geometric Morphometric Analysis of Interspecific and Intersexual Size and Shape Variation in Colophon Haughtoni Barnard, 1929, and C
Org Divers Evol DOI 10.1007/s13127-016-0289-z ORIGINAL ARTICLE Does size matter for horny beetles? A geometric morphometric analysis of interspecific and intersexual size and shape variation in Colophon haughtoni Barnard, 1929, and C. kawaii Mizukami, 1997 (Coleoptera: Lucanidae) Thea Eldred1 & Carlo Meloro2 & Clarke Scholtz3 & Declan Murphy1 & Katie Fincken1 & Matt Hayward4 Received: 28 December 2015 /Accepted: 17 May 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Colophon is an understudied, rare and endangered biological and phylogenetic insights and enabling species stag beetle genus with all species endemic to isolated moun- identification. tain peaks in South Africa’s Western Cape. Geometric mor- phometrics was used to analyse intersexual and interspecific Keywords Colophon . Lucanidae . Geometric variation of size and shape in the mandibles, heads, pronota morphometrics . Morphology . Species identification . and elytra of two sympatric species: Colophon haughtoni and Sexual dimorphism Colophon kawaii. All measured structures showed significant sexual dimorphism, which may result from male-male com- petition for females. Female mandibles were too small and Introduction featureless for analysis, but male Colophon beetles possess large, ornate mandibles for fighting. Males had significantly Colophon Gray, 1832, is a genus of endangered montane stag larger heads and pronota that demonstrated shape changes beetles (subfamily Lucaninae Latreille, 1804 (Kim and Farrell which may relate to resource diversion to the mandibles and 2015)) endemic to the Western Cape Province of South Africa their supporting structures. Females are indistinguishable (Fig. 1) (Geertsema and Owen 2007; Switala et al. 2015). The across species, but males were accurately identified using first species was described by Gray (1832) in 1832, but over mandibles, heads and pronota. -

Analysis of Foraging Behavior in Water Striders Jerry Su Abstract
Analysis of Foraging Behavior in Water Striders Jerry Su Abstract The foraging behaviors expressed by an individual may depend upon the state of that individual and local environmental condition. Understanding how abiotic conditions affect feeding behaviors may elucidate how animals choose where to forage and how much energy to expend on feeding. Water striders, Gerris remigis, are abundant insects that inhabit slow moving waters. I conducted two types of experiments on the water striders to (1) determine the effect of stream velocity on the preference of foraging habitat and (2) to examine whether the preference for location is controlled by current velocity or food availability. In the first set of experiments, the stream velocities were manipulated so that one side of the creek was faster than the other. I predicted that the water striders would tend to congregate in the swifter moving current because either instinctual behavior associated faster water current with a greater probability of obtaining food, or the side with greater flow carries more food items. This idea coincides with the rule of thumb decision making process. Results showed that there are consistently more water striders in the faster current. In the second set of experiments, I attempted to disentangle the effects of food and current. I predicted that the water striders would move over to the slower current when food availability increased to decrease the energy spent battling the current. Results show that there was movement towards the slower current with the food, but it was not statistically significant. These results allowed me to suggest an optimal foraging theory for the water strider.