Extraction and Characterization of Castor (Ricinus Communis) Seed Oil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tea Seed Oil and Health Properties Fatih Seyis1, Emine
Tea Seed Oil and Health Properties Fatih Seyis1, Emine Yurteri1, Aysel Özcan1 1Recep Tayyip Erdoğan University: Faculty of Agriculture and Natural Science, Field Crops Department, Rize/Turkey, e-mail: [email protected] Abstract: Tea Oil has a mild fragrant flavor that goes with anything. It’s not a heavy oil like Olive Oil, but thinner – more like almond oil. If the taste or “oiliness” of olive oil overpowers your food. Along with its mild taste and pleasant tea-like aroma, this oil touts impressive health benefits. Tea seed oil has a high smoke point, contains more monounsaturated fatty acids than olive oil, contains fewer saturated fatty acids than olive oil, contains high levels of Vitamin E, polyphenol antioxidants and both Omegas 3 and 6, but has less Omega 6 and Polyunsaturated Fats than olive oil. Health Benefits of tea seed oil are: it can be applied topically and consumed internally to obtain its health benefits, camellia oil can be used for skin, hair, has anti-cancer effects, effects boost immunity and reduces oxidative stress. Camellia oil is used for a variety of other purposes, for example for cooking, as machinery lubricant, as ingredient in beauty products like night creams, salves, in hair care products and perfumes and is used to coat iron products to prevent rusting. Key words: Tea, seed oil, health 1. Introduction Like other genera of Camellia (from Theaceae family), the tea plant (C.sinensis) produces large oily seeds. In some countries where tea seed oil is abundantly available, it has been accepted as edible oil (Sahari et al., 2004). -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

Olive Oil Jars Left Behind By
live oil jars left behind by the ancient Greeks are testament to our centuries- old use of cooking oil. Along with salt and pepper, oil Oremains one of the most important and versatile tools in your kitchen. It keeps food from sticking to pans, adds flavor and moisture, and conducts the heat that turns a humble stick of potato into a glorious french fry. Like butter and other fats, cooking oil also acts as a powerful solvent, unleashing fat-soluble nutrients and flavor compounds in everything from tomatoes and onions to spices and herbs. It’s why so many strike recipes begin with heating garlic in oil rather than, say, simmering it in water. The ancient Greeks didn’t tap many cooking oils. (Let’s see: olive oil, olive oil, or—ooh, this is exciting!—how about olive oil?) But you certainly can. From canola to safflower to grapeseed to walnut, each oil has its own unique flavor (or lack thereof), aroma, and optimal cooking temperature. Choosing the right kind for the task at hand can save you money, boost your health, and improve your cooking. OK, so you probably don’t stop to consider your cooking oil very often. But there’s a surprising amount to learn about What’s this? this liquid gold. BY VIRGINIAWILLIS Pumpkin seed oil suspended in corn oil—it looks like a homemade Lava Lamp! 84 allrecipes.com PHOTOS BY KATE SEARS WHERE TO store CANOLA OIL GRAPESEED OIL are more likely to exhibit the characteristic YOUR OIL flavor and aroma of their base nut or seed. -

Heating Olive Oil | the Olive Oil Source
Heating Olive Oil | The Olive Oil Source Search Log In / Register | Privacy Policy Checkout HOME STORE MAKING OLIVE OIL ENJOYING OLIVE OIL FACTS AND DEFINITIONS OLIVE PEOPLE OLIVE CHATTER Heating Olive Oil Heating Olive Oil and Smoke Point Myths about Cooking with Olive Oil HEATING OLIVE OIL AND SMOKE POINT One of the questions we are asked most often is what happens when olive oil is heated and/or used for frying. The important thing about cooking with any oil (olive or otherwise) is not to heat the oil over its smoke point (also referred to as smoking point). The smoke point refers to the temperature at which a cooking fat or oil begins to break down. The substance smokes or burns, and gives food an unpleasant taste. But what is the smoke point of olive oil? Depending on where you look for an answer, you may get vastly different ideas. Relationship between Smoke Point and Quality of Olive Oil The smoke point of oil varies with its quality. High quality extra virgin olive oils (with low free fatty acids) have a high smoke point. They are an excellent choice, but an expensive one. Mass produced, low quality olive oils have a much lower smoke point. At the Olive Oil Source, we believe that extra virgin olive oil smokes roughly between 400 and 365ºF (204 and 185ºC) depending on its free fatty acid content. Here is what the International Olive Oil Council (IOOC) has to say about frying food with olive oil: When heated, olive oil is the most stable fat, which means it stands up well to high frying temperatures. -

Good Food Fighter's Power PantryChecklist 2019
GOOD FOOD FIGHTER’S POWER PANTRY CHECKLIST 2019 COOKING & FLAVORING OILS 2 NUTS 3 NUT BUTTERS 3 SEEDS 4 SNACKY FOODS 4 DRIED FRUIT 5 PASTA 6 OTHER GRAINS 6 CANNED FISH 7 ANIMAL & PLANT BASED JERKY 7 BEANS 8 SOUP 8 SALAD DRESSING 8 OTHER 9 CONDIMENTS 9 HERBS 10 COFFEE 11 TEA 11 CHOCOLATE 12 WHERE TO SHOP 13 GoodFoodFighter.com facebook.com/goodfoodfighter • instagram @thegoodfoodfighter • [email protected] COOKING & FLAVORING OILS For cooking I use olive, coconut, avocado, and ghee. The higher the phenol level of olive oil, the higher the smoke point, and that will allow you to use for higher heat applications. I do not recommend frying with it. Macadamia nut oil has a very high smoke point. Macadamia, olive, flaxseed, black seed and pine nut oils are all great for salad dressings or drizzling on roast vegetables. They are all really healthy but have strong flavors so try experimenting with them. ◯ avocado oil Primal Kitchen Avocado Oil ◯ black seed oil Pure Indian Foods ◯ coconut oil Nutiva Organic Virgin Coconut Oil Dr. Bronners Organic Virgin Coconut Oil ◯ grass fed ghee Fourth and Heart Grass Fed Ghee Pure Indian Foods Coconut Ghee ◯ macadamia nut oil Brookfarm Premium Grade Macadamia Nut Oil ◯ olive oil Nunez de Prado Organic Extra Vigin Olive Oil Texas Hill Country Olive Oil Company Bragg Extra Virgin Olive Oil Rallis Ice Pressed Olive Oil Gaea Greek Extra Virgin Olive Oil Kasandrinos Extra Virgin Olive Oil McEvoy Organic Olive Oil ◯ pine nut oil Siberian Tiger Naturals Pine Nut Oil Power Pantry Checklist 2019 • GoodFoodFighter.com facebook.com/goodfoodfighter • instagram @thegoodfoodfighter • [email protected] NUTS Packed with vitamins and minerals, you can integrate them in almost any dish: salads, soups, eggs, oatmeal, yogurt, casseroles, meat rubs. -

Smoke Point of Common Oils
Smoke Point of Common Oils Almond Oil 430 F 221 C Matching the Correct Oil to the Right Cooking Method Avocado Oil: 520 F 271 C Cooking At, or Below 212 F Butter 300 F 150 C Low-temperature pan fry or poach Unrefined canola oil (smoke point is below 225 F) Canola Oil: 468 F 242 C Unrefined flaxseed oil (smoke point is below 225 F) Unrefined safflower oil (smoke point 225 F) Coconut Oil 350 F 177 C Unrefined sunflower oil (smoke point is below 225 F) Corn Oil: 457 F 236 C Cottonseed Oil 420 F 216 C Cooking from 212 F to 320 F For delicate, light Saute items Flaxseed Oil 225 F 107 C Unrefined corn oil (smoke point is below 32 F) Unrefined peanut oil (smoke point is below 320 F) Hazelnut Oil 430 F 221 C Semirefined safflower oil (smoke point is below 320 F) Unrefined soy oil (smoke point is below 320 F) Hemp Seed Oil 330 F 165 C Unrefined high-Oleic sunflower oil (smoke point is below 320 F) Grapeseed Oil 400 F 204 C Unrefined walnut oil (smoke point is below 320 F) Lard 360-400 F 140-200C Margarine 300 F 150C Cooking from 320 F to 450 F Macadamia Nut Oil 389 F 198 C High heat quick sauté, stir fry, or deep fry Mustard Oil 489 F 254 C Semirefined canola oil (smoke point is below 350 F) Refined canola oil (smoke point is below 400 F) Olive Oil: Extra Virgin 375 F 190 C Refined corn oil (smoke point is below 450 F) Unrefined olive oil (smoke point is below 320) Virgin 420 F 215 C Refined peanut oil (smoke point is below 450 F) Refined safflower oil (smoke point is below below 450 F) Olive Oil 438 F 225 C Unrefined sesame oil (smoke point is -

Camellia As an Oilseed Crop
HORTSCIENCE 52(4):488–497. 2017. doi: 10.21273/HORTSCI11570-16 Camellia as an Oilseed Crop Haiying Liang1 Department of Genetics and Biochemistry, Clemson University, Clemson, SC 29634 Bing-Qing Hao, Guo-Chen Chen, Hang Ye, and Jinlin Ma1 Guangxi Forestry Research Institute, Guangxi Key Laboratory of Non-wood Cash Crops Cultivation and Utilization, Nanning, P.R. China, 530002 Additional index words. biodiesel, cultivar, edible oil, new horticultural crop, oil camellias Abstract. Camellia is one of the four main oil-bearing trees along with olive, palm, and coconut in the world. Known as ‘‘Eastern Olive Oil,’’ camellia oil shares similar chemical composition with olive oil, with high amounts of oleic acid and linoleic acid and low saturated fats. Camellia was first exploited for edible oil in China more than 1000 years ago. Today, its oil serves as the main cooking oil in China’s southern provinces. Introduction of camellia oil into the Western countries was delayed until the recognition of its many health benefits. Although popularity for the oil has yet to grow outside of China, interest has emerged in commercial production of camellia oil in other countries in recent years. Unlike seed-oil plants that are grown on arable land, oil camellias normally grow on mountain slopes. This allows the new crop to take full usage of the marginal lands. To facilitate promoting this valuable crop as an alternative oil source and selecting promising cultivars for targeted habitats, this paper reviews the resources of oil camellias developed in China, use of by-products from oil-refining process, as well as the progress of developing camellias for oil production in China and other nations. -

Healthy Fats + Oils
Healthy Fats + Oils Popular to what we used to believe a few years back good quality fat plays a huge role in our cellular health. Our cells have a membrane made un of a bi-layer of lipids (fats), how permeable and good this membrane is will determine if toxins come out and nutrients come into the cell amount other functions. When eating good fats, specially unadulterated omega 6, and a good ratio of Omega 3 and 6 will help us maintain that bi-layer optimal and flexible. Some healthy fats we can incorporate into our diets are: - Avocado - Olives - Nuts (walnuts, brazil nuts, pine nuts, hazelnuts, pecans, macadamia, almonds, cashews) - Seeds (hemp, pumpkin, sunflower, sesame, chia, flax) - Oils (avocado, olive, walnut, macadamia, camelina, coconut, walnut, flax, pumpkin) - Animal fats (lard, tallow) - Coconut meat Some oils are meant to be used for cooking and some are better suited for no heat. Knowing what oil to use depending on what we are cooking is key to getting the best nutritional value. When oils surpass their smoke point (maximum heat temperature) they will burn and become damaged. Its been said it takes our body up to 80 days to clear our damaged oils! Here is a guide to the oils we should be using and their smoke point as well as the best uses for them. Fat/Oil Smoke Point Best Uses Avocado oil 520 F High heat cooking, low heat cooking, dressing Butter 300 F High heat cooking, baking Camelina oil 475 F High heat cooking, sautéing Coconut oil 350 F High heat cooking, sauteing, baking Duck fat 375 F High heat cooking Flax seed oil 225 F Dressing, smoothies, best kept in fridge. -
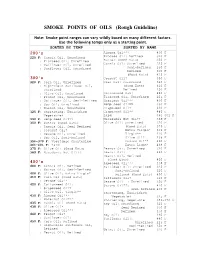
SMOKE POINTS of OILS (Rough Guideline)
SMOKE POINTS OF OILS (Rough Guideline) Note: Smoke point ranges can vary wildly based on many different factors. Use the following temps only as a starting point. SORTED BY TEMP SORTED BY NAME 200's Almond Oil*** 430 F 225 F: Canola Oil, Unrefined Avocado Oil: Refined 520 F : Flaxseed Oil, Unrefined Butter (Good Eats) 350 F : Safflower Oil, Unrefined Canola Oil: Unrefined 225 F : Sunflower Oil, Unrefined Semi-Refined 350 F Refined 400 F (Good Eats) 435 F 300's Coconut Oil† 350 F 320 F: Corn Oil, Unrefined Corn Oil: Unrefined 320 F : High-Oleic Sunflower Oil, (Good Eats) 410 F Unrefined Refined 450 F : Olive Oil, Unrefined Cottonseed Oil† 420 F : Peanut Oil, Unrefined Flaxseed Oil, Unrefined 225 F : Safflower Oil, Semi-Refined Hazelnut Oil*** 430 F : Soy Oil, Unrefined Hemp Seed Oil¥¥ 330 F : Walnut Oil, Unrefined Grapeseed Oil¥ 420 F 325 F: Shortening, Emulsified Grapeseed Oil** 485 F Vegetable† Lard 361-401 F 330 F: Hemp Seed Oil¥¥ Macadamia Nut Oil†† 389 F 350 F: Butter (Good Eats) Olive Oil: Unrefined 320 F : Canola Oil, Semi-Refined (Good Eats) 375 F : Coconut Oil† Extra Virgin* 406 F : Sesame Oil, Unrefined Virgin** 420 F : Soy Oil, Semi-Refined Olive Oil* 438 F 356-370 F: Vegetable Shortening Pomace Oil** 460 F 361-401 F: Lard Extra Light* 468 F 375 F: Olive Oil (Good Eats) Peanut Oil: Unrefined 320 F 389 F: Macadamia Nut Oil†† Peanut Oil† 440 F Peanut Oil, Refined (Good Eats) 450 F 400's Rapeseed Oil*** 438 F 400 F: Canola Oil, Refined Safflower Oil: Unrefined 225 F : Walnut Oil, Semi-Refined Semi-Refined 320 F 406 F: Olive Oil, -

Frying Oils: Selection, Smoke Points and Potential Deleterious Effects for Health
REVIEW Frying oils: selection, smoke points and potential deleterious effects for health Laurence Eyres, FNZIFST Frying is a complex, high temperature process with many Of the industrial and commercial fats, palm olein (48% saturates) is reactions of hydrolysis, oxidation and polymerisation occurring probably the most economic and readily available globally. Palm during the frying life of the oil with resultant effects on the fried has come under fire for its saturated fat content and environmental food with respect to texture, taste, shelf-life and nutritional sustainability. It is also semi-solid in temperate climates and many properties. users look for liquid oils. Those that are highly suitable may include high oleic varieties of sunflower oil, canola oil and safflower oil. High Deep fat frying and shallow pan frying are very common ways of oleic acid canola is currently being grown in Canterbury by Pure Oil cooking and dehydrating a wide range of foods at temperatures from Ltd. 170-190 °C. Deep frying is generally carried out between 170-180°C The specifications, functional requirements and supply conditions for and shallow pan frying tends to be uncontrolled with temperatures the industrial food market are far more rigorous and demanding than between 180-230°C. for the retail market. Some specialty oils supplied to the industry are Probably the most common fried snack products are potato and corn not available at retail. chips. Potatoes are cooked from a moisture of 80% down to 1.5 %. The fat contents of fried foods vary from around 35% in potato chips to 7-14% in French fries. -

Approximately 40% of a Live Beef Animal Weight Is Processed Through a Rendering Plant
Chapter 5. Edible Rendering-- Rendered Products for Human Use In “Essential Rendering” Dr. Herb Ockerman and Lopa Basu Ohio State University Summary This chapter focuses on animal by products used directly by humans. Preliminary information on world and U.S. meat and by-product production is discussed. The main products focused upon are fats and oils and their properties, animal by-products that are harvested from the carcass and cooked by the consumer or incorporated into consumable food items. Also discussed is gelatin extraction, edible tissue separated from bone, and medical and pharmaceutical products for human treatment. Where appropriate, references are given where more detailed information is available. Definitions are important to this industry and some of the critical ones to edible animal by-products are: Batch cooker – Horizontal, steam-jacketed cylinder with a mechanical agitator Centrifuge – Machine using centrifugal force to separate materials of different densities Chitlings - Small intestines of hogs Continuous cooker – The flow of material through the system is constant 1 Cracklings – Solid protein material from screw press or cooking of pork fat after removal of lard Dry rendering – Releasing fat by dehydration Edible – Products for human consumption which is under the inspection of USDA/FSIS (for meat items in the U.S.) Giblets – Consists of the neck, liver, heart, and gizzard of poultry Grease – Fats with lower melting points, softer. Titer less than 40ºC Lard (grease) – Fat from hogs (porcine), softer t than fat from ruminants Rendering – Fatty or oil materials in meat is melted away or cooked from the solid portion of the animal tissue Tallow – Fat from beef (bovine), mutton (ovine), goat (capra), camel (camelus), llama (lama), deer (cervidae). -

Smoke Point Testing of Cooking Oil
SNA Research Conference Vol. 59 2014 Engineering, Structures and Innovations Scot Langolis Section Editor Engineering, Structures and Innovations 292 SNA Research Conference Vol. 59 2014 Smoke Point Testing of Tea-seed Oil (Camellia oleifera) Charles B Allen, Jr, John M. Ruter Department of Horticulture, 1111 Miller Plant Sciences Building Athens, GA 30602-7273 [email protected] Index Words: Camellia, oleifera, cooking oil, smoke point Significance to Industry: Camellia oleifera, an oilseed crop native to Asia, has recently been adapted for production in the Southeastern United States. The oil derived from the seed, known as tea-seed oil, is a popular cooking oil in its native regions and is known to have a relatively high smoke point. The significance of the smoke point is that when an oil reaches this temperature it degrades and increases free radical production [9]. Oxygen derived free radicals are thought to be related to the formation of cancer, inflammation, atherosclerosis, ischemia-reperfusion injuries, aging, Alzheimer’s disease, shock, diabetes, cataracts, hypertension, cardiovascular disease, exercise related muscle damage, infertility and other pathological changes [4]. There are an abundance of claims on the smoke point temperatures of various cooking oils, however, little work has been conducted by research institutions on these smoke points and little to no research on tea-seed oil specifically. In this study, smoke point testing was conducted on Camellia oil as well as several popular commercial cooking oils. Based on accepted food standards, the smoke point of tea-seed oil allows it to be classified as an edible oil with high heat tolerance. Nature of Work: Camellia is a genus of evergreen flowering trees and shrubs native to China, Japan, and other regions of Southeast Asia.