WHO Drug Information Vol. 02, No. 4, 1988
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Teclozan/Tryparsamide
Teclozan/Tryparsamide 849 Pharmacokinetics Children are given 50 to 60 mg/kg daily for 3 or 5 days Ciplox TZ; Ciptini; Citizol; Entrolate†; Forcan TZ; Genflox TZ; Helipac; Nor T; Norflox TZ; Normax TZ; Ofler-TZ; Oflox TZ; Olfi TZ; OTC HP Kit; The pharmacokinetics of tinidazole resemble those of respectively. Parabact; Pylokit; Tinidafyl Plus; Tinvista-CF; Tinvista-NF; Wotinex; Indon.: metronidazole although the half-life is longer. Fasigyn-Nystatin; Ital.: Fasigin N; Malaysia: Pylobact Combi; Mex.: Afu- A single dose of tinidazole 2 g is given orally in the mix; Fasigyn VT; Mebeciclol; Rus.: Pylobact (Пилобакт). Tinidazole is rapidly and almost completely absorbed treatment of giardiasis, trichomoniasis, and acute after oral doses and, typically, a peak plasma concen- necrotising ulcerative gingivitis; 50 to 75 mg/kg as a tration of about 40 micrograms/mL is achieved 2 hours single dose is given to children with giardiasis or tri- Toltrazuril (BAN, USAN, rINN) after a single 2-g dose, falling to about chomoniasis. It may sometimes be necessary to repeat Bay-Vi-9142; Toltrazurilo; Toltrazurilum. 1-Methyl-3-(4-{p-[(trif- 10 micrograms/mL at 24 hours and this dose once. In trichomoniasis, sexual partners luoromethyl)thio]phenoxy}-m-tolyl)-s-triazine-2,4,6(1H,3H,5H)- 2.5 micrograms/mL at 48 hours; concentrations above should also be treated. trione. 8 micrograms/mL are maintained by daily mainte- In bacterial vaginosis, a single 2-g dose of tinidazole Тольтразурил nance doses of 1 g. Comparable concentrations are is usually given orally, although higher cure rates have C18H14F3N3O4S = 425.4. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -
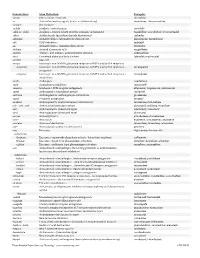
Downloaded from Survive Nursing | Survivenursing.Com V20110426
Generic Stem Stem Definition Examples -abine (see -arabine, -citabine) decitabine -ac Anti-inflammatory agents (acetic acid derivatives) bromfenac; dexpemedolac -acetam See -racetam -actide Synthetic corticotropins seractide -adol or -aldol- Analgesics (mixed opiate receptor agonists/ antagonists) tazadolene; spiradolene; levonantradol -adox Antibacterials (quinoline dioxide derivatives) carbadox -afenone Antiarrhythmics (propafenone derivatives) alprafenone; diprafenone -afil PDE5 inhibitors tadalafil -aj- Antiarrhythmics (ajmaline derivatives) lorajmine -aldrate Antacid aluminum salts magaldrate -algron Alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol Combined alpha and beta blockers labetalol; medroxalol -amivir (see -vir) -ampa Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) -ampanel Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; becampanel antagonists -ampator Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; forampator modulators -andr- Androgens nandrolone -anib Angiogenesis inhibitors semaxanib -anserin Serotonin 5-HT2 receptor antagonists altanserin; tropanserin; adatanserin -antel Anthelmintics (undefined group) carbantel -antrone Antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine Antineoplastics (arabinofuranosyl derivatives) fazarabine; fludarabine aril-, -aril, -aril- Antiviral (arildone derivatives) pleconaril; arildone; fosarilate -arit Antirheumatics (lobenzarit type) lobenzarit; clobuzarit -arol -

C:\Myfiles\Linda's Work\Houchens\WA 2-6
1 2 This page intentionally left blank. 3 4 Draft Steroidogenesis DRP 110 May 2002 1 6.0 DEVELOPMENTAL STATUS OF THE ASSAY AND RECOMMENDATIONS 2 FOR PREVALIDATION STUDIES 3 4 6.1 Current Status 5 6 The endpoint included in the sectioned testes assay has been evaluated in other studies. 7 However, the protocol itself has not been validated. Pending a final decision on the study 8 design, the protocol would be ready to enter the prevalidation phase. 9 10 6.2 Recommendation for Optimization of the Sectioned Testis Assay Protocol 11 12 6.2.1 Testicular Preparation Issues 13 14 Optimization of the assay could be determined for the amount of testis actually needed to 15 obtain a given level of sensitivity. For example, a single testis from an adult SD rat weighs 16 approximately 1 g. If such a testis were quarter sectioned, then each section would weigh 17 approximately 250 mg, which is the weight of the sample generally described by investigators 18 who have used quartered sections of testis. However, no documentation was found that 19 demonstrates whether smaller sections would give similar results. Thus, it would be 20 advantageous to conduct a study that investigates whether the sensitivity of the preparation is 21 affected by the amount of testicular tissue used and, if so, if there is an optimal and/or threshold 22 amount to use. The weight of the sections to be tested could range from the customary amount 23 used, i.e., 250 mg, down to an amount of tissue that represents a practical minimum, e.g., 5 to 24 10 mg. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Download Author Version (PDF)
RSC Advances This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. This Accepted Manuscript will be replaced by the edited, formatted and paginated article as soon as this is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/advances Page 1 of 84 RSC Advances Pyrrole: A Resourceful Small Molecule in Key Medicinal Hetero-aromatics Varun Bhardwaj a, *, Divya Gumber b, Vikrant Abbot a, Saurabh Dhiman a, Poonam Sharma a aPharmaceutical Chemistry Laboratory, Department of Biotechnology, Bioinformatics and Pharmacy, Jaypee University of Information Technology, Waknaghat, Solan, Himachal Pradesh, 173234 India. bDepartment of Pharmaceutical Chemistry, Banasthali Vidyapith, Banasthali, Rajasthan, 304022, India. Manuscript Accepted Advances RSC Corresponding Author (*) Dr. Varun Bhardwaj, Tel: 91-94189-05865 Email: [email protected] ; [email protected] 1 RSC Advances Page 2 of 84 Abstract Pyrrole is widely known as biologically active scaffold which possess diverse nature of activities. -

WO 2016/033635 Al 10 March 2016 (10.03.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2016/033635 Al 10 March 2016 (10.03.2016) P O P C T (51) International Patent Classification: AN, Martine; Epichem Pty Ltd, Murdoch University Cam Λ 61Κ 31/155 (2006.01) C07D 249/14 (2006.01) pus, 70 South Street, Murdoch, Western Australia 6150 A61K 31/4045 (2006.01) C07D 407/12 (2006.01) (AU). ABRAHAM, Rebecca; School of Animal and A61K 31/4192 (2006.01) C07D 403/12 (2006.01) Veterinary Science, The University of Adelaide, Adelaide, A61K 31/341 (2006.01) C07D 409/12 (2006.01) South Australia 5005 (AU). A61K 31/381 (2006.01) C07D 401/12 (2006.01) (74) Agent: WRAYS; Groud Floor, 56 Ord Street, West Perth, A61K 31/498 (2006.01) C07D 241/20 (2006.01) Western Australia 6005 (AU). A61K 31/44 (2006.01) C07C 211/27 (2006.01) A61K 31/137 (2006.01) C07C 275/68 (2006.01) (81) Designated States (unless otherwise indicated, for every C07C 279/02 (2006.01) C07C 251/24 (2006.01) kind of national protection available): AE, AG, AL, AM, C07C 241/04 (2006.01) A61P 33/02 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C07C 281/08 (2006.01) A61P 33/04 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, C07C 337/08 (2006.01) A61P 33/06 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, C07C 281/18 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (21) International Application Number: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PCT/AU20 15/000527 PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (22) International Filing Date: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 28 August 2015 (28.08.2015) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Cortisol, Pregnene and Pregnane Profiles in Normal and Dysmature Newborn Pony Andlighthorse Foals
AN ABSTRACT OF THE THESIS OF Bernadette E. Voller for the degree of Master of Science in Animal Science presented on April 15, 1993. Title: Cortisol, Pregnene and Pregnane Profiles in Normal and Dysmature Newborn Pony andLighthorse Foals. Abstract approved: Redacted for Privacy In order to study normal and abnormalprogestin biosynthesis, 15 newborn lighthorse and pony foals(325 to 358 d gestation) were either givensynthetic ACTH (.2 IU/kg BW, i.v., n = 3), ACTH togetherwith a 3B- hydroxysteroid dehydrogenase blocker (trilostane) (10 mg/kg BW, p.o., n = 7), or no treatment (n = 5) at 4 h of age. Plasma samples were taken from birth through 96h of age and assayed for cortisolby radioimmunoassay, and for pregnenolone, 5-pregnene-36,20B-diol (P5BB), 5-pregnene- 38,20a-diol (P5Ba), progesterone, 5a-pregnane-38,20B-diol (5a-DHP-BB), 5a-pregnane-38,20a-diol (5a-DHP-Ba),38- hydroxy-5a-pregnan-20-one (38-0H-5a-DHP) and 20B-hydroxy- 4-pregnen-3-one (20B-P4) by gas chromatography/mass spectrometry. Preliminary comparisons demonstrated no significant differences in cortisol or progestin concentrations between normal foals given ACTH and ACTH/trilostane treatments and these groups werecombined for statistical purposes. In normal foals cortisol increased from birth (99.54 ± 7.44 ng/ml, mean ± SE) to maximum (124.18 ± 28.89 ng/ml) within 1 h of birth and rapidly declined, increasing again if treated with ACTH (129.04 ± 14.23 vs 24.11 ± 2.72 ng/ml at 6 h, for ACTH and normals, respectively)(P < .05); cortisol was below 11 ng/ml by 48 h.