Prioritization of Health Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Classification of Caroli's Disease: an Analysis of 30 Patients
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector DOI:10.1111/hpb.12330 HPB ORIGINAL ARTICLE Clinical classification of Caroli's disease: an analysis of 30 patients Zhong-Xia Wang1,2*, Yong-Gang Li2*, Rui-Lin Wang2, Yong-Wu Li3, Zhi-Yan Li3, Li-Fu Wang2, Hui-Ying Yang2, Yun Zhu2, Yao Wang2, Yun-Feng Bai2, Ting-Ting He2, Xiao-Feng Zhang2 & Xiao-He Xiao1,2 1Department of Graduate School, 301 Hospital, 2Integrative Medical Centre, and 3Imaging Centre, 302 Hospital, Beijing, China Abstract Background: Caroli's disease (CD) is a rare congenital disorder. The early diagnosis of the disease and differentiation of types I and II are of extreme importance to patient survival. This study was designed to review and discuss observations in 30 patients with CD and to clarify the clinical characteristics of the disease. Methods: The demographic and clinical features, laboratory indicators, imaging findings and pathology results for 30 patients with CD were reviewed retrospectively. Results: Caroli's disease can occur at any age. The average age of onset in the study cohort was 24 years. Patients who presented with symptoms before the age of 40 years were more likely to develop type II CD. Approximately one-third of patients presented without positive signs at original diagnosis and most of these patients were found to have type I CD on pathology. Anaemia, leucopoenia and thrombocytopoenia were more frequent in patients with type II than type I CD. Magnetic resonance cholangiopancreatography (MRCP) and computed tomography (CT) examinations were most useful in diagnosing CD. -

DENTIN HYPERSENSITIVITY: Consensus-Based Recommendations for the Diagnosis & Management of Dentin Hypersensitivity
October 2008 | Volume 4, Number 9 (Special Issue) DENTIN HYPERSENSITIVITY: Consensus-Based Recommendations for the Diagnosis & Management of Dentin Hypersensitivity A Supplement to InsideDentistry® Published by AEGISPublications,LLC © 2008 PUBLISHER Inside Dentistry® and De ntin Hypersensitivity: Consensus-Based Recommendations AEGIS Publications, LLC for the Diagnosis & Management of Dentin Hypersensitivity are published by AEGIS Publications, LLC. EDITORS Lisa Neuman Copyright © 2008 by AEGIS Publications, LLC. Justin Romano All rights reserved under United States, International and Pan-American Copyright Conventions. No part of this publication may be reproduced, stored in a PRODUCTION/DESIGN Claire Novo retrieval system or transmitted in any form or by any means without prior written permission from the publisher. The views and opinions expressed in the articles appearing in this publication are those of the author(s) and do not necessarily reflect the views or opinions of the editors, the editorial board, or the publisher. As a matter of policy, the editors, the editorial board, the publisher, and the university affiliate do not endorse any prod- ucts, medical techniques, or diagnoses, and publication of any material in this jour- nal should not be construed as such an endorsement. PHOTOCOPY PERMISSIONS POLICY: This publication is registered with Copyright Clearance Center (CCC), Inc., 222 Rosewood Drive, Danvers, MA 01923. Permission is granted for photocopying of specified articles provided the base fee is paid directly to CCC. WARNING: Reading this supplement, Dentin Hypersensitivity: Consensus-Based Recommendations for the Diagnosis & Management of Dentin Hypersensitivity PRESIDENT / CEO does not necessarily qualify you to integrate new techniques or procedures into your practice. AEGIS Publications expects its readers to rely on their judgment Daniel W. -

Biliary Tract
2016-06-16 The role of cytology in management of diseases of hepatobiliary ducts • Diagnosis in patients with radiologically/clinically detected lesions • Screening of dysplasia/CIS/cancer in risk groups biliary tract cytology • Preoperative evaluation of the candidates for liver transplantation (Patients with cytological low-grade and high-grade Mehmet Akif Demir, MD dysplasia/adenocarcinoma are currently referred for liver transplantation Sahlgrenska University Hospital in some institutions). Gothenburg Sweden Sarajevo 18th June 2016 • Diagnosis of the benign lesions and infestations False positive findings • majority of false positive cases have a Low sensitivity but high specificity! background of primary sclerosing cholangitis. – lymphoplasmacytic sclerosing pancreatitis and cholangitis, – primary sclerosing cholangitis, – granulomatous disease, – non-specific fibrosis/inflammation – stone disease. False negative findings • Repeat brushing increases the diagnostic yield and should be performed when sampling • Poor sampling biliary strictures with a cytology brush at ERCP. • Lack of diagnostic criteria for dysplasia-carcinoma in situ • Difficulties in recognition of special tumour types – well-differentiated cholangiocarcinoma with tubular architecture • Predictors of positive yield include – gastric foveolar type cholangiocarcinoma with mucin-producing – tumour cells. older age, •Underestimating the significance of the smear background – mass size >1 cm, and – stricture length of >1 cm. •The causes of false negative cytology –sampling -

Dance/Movement Therapy with Children Adopted out of Foster Care
Sarah Lawrence College DigitalCommons@SarahLawrence Dance/Movement Therapy Theses Dance/Movement Therapy Graduate Program 5-2019 Dance of Attachment: Dance/Movement Therapy with Children Adopted Out of Foster Care Megan Haase Sarah Lawrence College Follow this and additional works at: https://digitalcommons.slc.edu/dmt_etd Part of the Dance Movement Therapy Commons Recommended Citation Haase, Megan, "Dance of Attachment: Dance/Movement Therapy with Children Adopted Out of Foster Care" (2019). Dance/Movement Therapy Theses. 50. https://digitalcommons.slc.edu/dmt_etd/50 This Thesis - Open Access is brought to you for free and open access by the Dance/Movement Therapy Graduate Program at DigitalCommons@SarahLawrence. It has been accepted for inclusion in Dance/Movement Therapy Theses by an authorized administrator of DigitalCommons@SarahLawrence. For more information, please contact [email protected]. Running Head: DANCE OF ATTACHMENT 1 Dance of Attachment: Dance/Movement Therapy with Children Adopted Out of Foster Care Megan Haase Submitted in partial completion of the Master of Science Degree at Sarah Lawrence College May 2019 DANCE OF ATTACHMENT 2 Abstract This thesis is an investigative inquiry into the use of dance/movement therapy with children adopted out of foster care and their families. By focusing on the relevance of attachment and trauma with this population, this thesis draws connections between dance/movement therapy theoretical components and literature and the needs of this population. Children adopted out of foster care experience disrupted attachments and traumatic histories of separation, abuse, and neglect. As the literature illustrates, attachment and trauma are body-level experiences, and when addressing these issues, they must be approached through a modality that involves, engages, and values the body and the memories, interactions, and healing potential that it holds. -

Epidemiology and Indices of Gingival and Periodontal Disease Dr
PEDIATRIC DENTISTRY/Copyright ° 1981 by The American Academy of Pedodontics Vol. 3, Special Issue Epidemiology and indices of gingival and periodontal disease Dr. Poulsen Sven Poulsen, Dr Odont Abstract Validity of an index indicates to what extent the This paper reviews some of the commonly used indices index measures what it is intended to measure. Deter- for measurement of gingivitis and periodontal disease. mination of validity is dependent on the availability Periodontal disease should be measured using loss of of a so-called validating criterion. attachment, not pocket depth. The reliability of several of Pocket depth may not reflect loss of periodontal the indices has been tested. Calibration and training of attachment as a sign of periodontal disease. This is be- examiners seems to be an absolute requirement for a cause gingival swelling will increase the distance from satisfactory inter-examiner reliability. Gingival and periodontal disease is much more severe in several the gingival margin to the bottom of the clinical populations in the Far East than in Europe and North pocket (pseudo-pockets). Thus, depth of the periodon- America, and gingivitis seems to increase with age resulting tal pocket may not be a valid measurement for perio- in loss of periodontal attachment in approximately 40% of dontal disease. 15-year-old children. Apart from the validity and reliability of an index, important factors such as the purpose of the study, Introduction the level of disease in the population, the conditions under which the examinations are going to be per- Epidemiological data form the basis for planning formed etc., will have to enter into choice of an index. -

The Joy Dance Specific Effects of a Single Dance Intervention On
The Arts in Psychotherapy 34 (2007) 340–349 The joy dance Specific effects of a single dance intervention on psychiatric patients with depression Sabine C. Koch, PhD, MA a,∗, Katharina Morlinghaus b, Thomas Fuchs, PhD, MD b a University of Heidelberg, Department of Psychology, Hauptstr. 47-51, 60117 Heidelberg, Germany b Psychiatric University Hospital Heidelberg, Heidelberg, Germany Abstract This study investigated the specific effects of a dance intervention on the decrease of depression and the increase of vitality and positive affect in 31 psychiatric patients with main or additional diagnosis of depression. Patients participated in one of three conditions: a dance group performing a traditional upbeat circle dance, a group that listened just to the music of the dance (music only), and a group that moved on a home trainer bike (ergometer) up to the same level of arousal as the dance group (movement only). While all three conditions alleviated or stabilized the condition of the patients, results suggest that patients in the dance group profited most from the intervention. They showed significantly less depression than participants in the music group (p < .001) and in the ergometer group (p < .05), and more vitality (p < .05) than participants in the music group on post-test self-report scales immediately after the intervention. Stimulating circle dances can thus have a positive effect on patients with depression and may be recommended for use in dance/movement therapy and other complementary therapies. © 2007 Elsevier Inc. All rights reserved. Keywords: Embodiment; Kestenberg Movement Profile (KMP); Circle dance; Depression; Clinical evaluation; Joy; Dance/movement therapy Depression is affecting about 121 million people worldwide (WHO, 2007). -

Vasculitis: Pearls for Early Diagnosis and Treatment of Giant Cell Arteritis
Vasculitis: Pearls for early diagnosis and treatment of Giant Cell Arteritis Mary Beth Humphrey, MD, PhD Professor of Medicine McEldowney Chair of Immunology [email protected] Office Phone: 405 271-8001 ext 35290 October 2019 Relevant Disclosure and Resolution Under Accreditation Council for Continuing Medical Education guidelines disclosure must be made regarding relevant financial relationships with commercial interests within the last 12 months. Mary Beth Humphrey I have no relevant financial relationships or affiliations with commercial interests to disclose. Experimental or Off-Label Drug/Therapy/Device Disclosure I will be discussing experimental or off-label drugs, therapies and/or devices that have not been approved by the FDA. Objectives • To recognize early signs of vasculitis. • To discuss Tocilizumab (IL-6 inhibitor) as a new treatment option for temporal arteritis. • To recognize complications of vasculitis and therapies. Professional Practice Gap Gap 1: Application of imaging recommendations in large vessel vasculitis Gap 2: Application of tocilizimab in treatment of giant cell vasculitis Cranial Symptoms Aortic Vision loss Aneurysm GCA Arm PMR Claudication FUO Which is not a risk factor or temporal arteritis? A. Smoking B. Female sex C. Diabetes D. Northern European ancestry E. Age Which is not a risk factor or temporal arteritis? A. Smoking B. Female sex C. Diabetes D. Northern European ancestry E. Age Giant Cell Arteritis • Most common form of systemic vasculitis in adults – Incidence: ~ 1/5,000 persons > 50 yrs/year – Lifetime risk: 1.0% (F) 0.5% (M) • Cause: unknown At risk: Women (80%) > men (20%) Northern European ancestry>>>AA>Hispanics Age: average age at onset ~73 years Smoking: 6x increased risk Kermani TA, et al Ann Rheum Dis. -
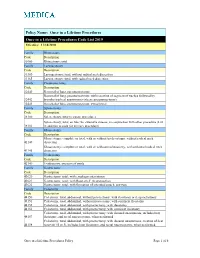
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Pattern of Arterial Involvement Ofthe
368 BritishJournal ofOphthalmology, 1991, 75, 368-371 CASE REPORTS Br J Ophthalmol: first published as 10.1136/bjo.75.6.368 on 1 June 1991. Downloaded from Pattern of arterial involvement of the head, neck, and eyes in giant cell arteritis: three case reports Z Butt, J F Cullen, E Mutlukan Abstract arteries at necropsy showed characteristic The findings oftwo post-mortem examinations changes ofGCA (see below). and one CT scan ofpatients with biopsy proved Post-mortenfindings. Macroscopically the main giant cell arteritis (GCA) are presented. The arteries at the base ofthe brain were virtually free presence or absence of intracranial involve- from atheroma, but a plug ofrather firm clot was ment in GCA is discussed. present in the stump ofthe right internal carotid. The circle of Willis was normally constituted. Several haemorrhagic infarcts were noted in the Giant cell arteritis (GCA) is rarely fatal, and cerebrum (frontal, parietal, temporal, and references to the condition in post-mortem occipital lobes) and cerebellum. Microscopic material are uncommon.'-16 However, this may examination confirmed that these were be related to non-recognition of a fatal outcome very recent, practically terminal, infarcts. in patients with GCA and because post-mortem Occasionally small meningeal arteries overlying examinations of elderly patients dying from the cortical infarcts were noted to contain recent vascular disorders are not routinely carried out. thrombus, but in none of the sections was there GCA may be concealed among the cases diag- evidence ofgiant cell arteritis. nosed as ischaemic catastrophes due to arterio- Sections of the temporal, ophthalmic, verte- sclerosis. " bral, internal carotid (in neck), external carotid, Patients suffering from GCA have a range of left common carotid, and coronary arteries symptoms including headache, jaw claudication, showed changes typical of giant cell arteritis. -

Pro-Argin, a Breakthrough Technology Based Upon Arginine
American Journal of Dentistry, Vol. 22, Special Issue A, March, 2009 A, March, 22, Special Issue Vol. American Journal of Dentistry, Vol. 22, Special Issue A, March, 2009 - p. 1A - 24A Introducing Pro-Argin™ A Breakthrough Technology Based upon Arginine and Calcium for In-Office Treatment of Dentin Hypersensitivity _______________________________________________________________________________________________________________________________________________________________ Editorial _______________________________________________________________________________________________________________________________________________________________ Dentin hypersensitivity: Beneficial effects of an arginine-calcium carbonate desensitizing paste Dentin hypersensitivity is a common occurrence diately after dental scaling procedures and its and is often a chief concern among patients. The sustained relief over 4 weeks. Another paper pre- pain associated with dentin hypersensitivity is sents the results of a double-blind, stratified, caused by some type of external stimulus and the randomized clinical study showing the successful sensitivity can range in its intensity from patient to desensitizing effect of the 8% arginine-calcium patient. The successful management of dentin carbonate paste tested, when applied as a pre- hypersensitivity is often very challenging for the procedure to professional dental cleaning. dental professional. The cause of the pain and the This Special Issue also includes a study con- description of the discomfort reported by -

An Exmanination of Dance/Movement
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Drexel Libraries E-Repository and Archives AN EXAMINATION OF DANCE/MOVEMENT THERAPISTS’ APPROACHES TO THE TREATMENT OF SUBSTANCE ABUSE/ALCOHOLIC POPULATION: AN INTERVIEW STUDY Thesis Presented to The Faculty of the College of Nursing and Health Professions Drexel University In Partial Fulfillment Of the Requirements for the Degree Master of Arts By Kristin Tillotson, B.F.A Hahnemann Creative Arts in Therapy Program Dance/Movement Therapy December 2007 ABSTRACT The average alcohol consumption for Americans over the age of 14 is three gallons of pure alcohol per person per year. The lifetime prevalence of alcoholism in the United States is about thirteen percent in the United States (Volpicelli 1997). Alcohol and illegal drug use often have close ties with one another. In 2004 the amount of alcohol consumption was strongly associated with illicit drug use in 2004 (SAMHSA). Consequently, finding effective treatment approaches present important challenges for therapists various modalities. The purpose of this study is to examine how Dance/Movement Therapists utilize and adapt Dance/Movement Therapy (D/MT) techniques and interventions in treating this population. The secondary purpose of this thesis was to examine how Dance/Movement Therapists integrate existing substance abuse treatment models and support systems into their own clinical work. Current research and literature in the Dance/Movement Therapy field that identify and/or clarify current treatment approaches in substance abuse and alcoholism are limited. The research design that best fit the research question stated above was a collective case study. -

DANCE/MOVEMENT THERAPY and the PSYCHOSOCIAL WELL-BEING of LEARNERS with VISUAL IMPAIRMENT: a CASE STUDY by MICHELLE BOTHA Subm
DANCE/MOVEMENT THERAPY AND THE PSYCHOSOCIAL WELL-BEING OF LEARNERS WITH VISUAL IMPAIRMENT: A CASE STUDY by MICHELLE BOTHA submitted in accordance with the requirements for the degree of MASTER OF EDUCATION School Guidance and Counselling at the UNIVERSITY OF SOUTH AFRICA SUPERVISOR: Prof. D. Kruger November 2018 ii DECLARATIONS Name: Michelle Botha Student number: 49923846 Degree: Master of Education (School Guidance and Counselling) I declare that Dance/Movement Therapy and the psychosocial well-being of learners with visual impairment: A case study is my own work and that all the sources I have used or quoted have been indicated or acknowledged by means of complete references. I further declare that I submitted the dissertation to originality checking software and that it falls within the accepted parameters for originality. ________________________ 15 November 2018 SIGNATURE DATE I, Professor Deirdré Krüger, hereby declare that I perused all the originality checking software reports and I confirm that the dissertation meets a high standard of originality. 30 October 2018 Deirdré Krüger Date Supervisor iii ACKNOWLEDGEMENTS I would like to express my sincere gratitude to the individuals who assisted, guided and supported me in this research endeavour: o My Heavenly Father, my Provider, Eraser of writer’s block and Source of energy and inspiration. o Professor Deirdré Kruger, not only for her expert guidance and commitment, but also for her concern, encouragement and support. o My husband, Jaco Botha, for his endless patience, constant emotional support and encouragement and countless after-midnight cups of coffee. Ek het jou lief soos die Kaap, my Engel. o My mom, Berenice Kleynhans, who made so many sacrifices as a single mother so that I could not only finish my pre-graduate studies, but also continue with the post- graduate qualifications which lead up to my Master’s degree.