EI Research Report Hazard Analysis for Offshore Carbon Capture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Review 2014 Incorporating the Annual Report and Accounts of the Institution of Chemical Engineers
ADVANCING CHEMICAL ENGINEERING WORLDWIDE Annual Review 2014 Incorporating the annual report and accounts of the Institution of Chemical Engineers Chemical engineers creating, maintaining and improving quality of life Shining a light on chemical engineering At the start of my presidential year, I made three commitments. First, I stressed the need for chemical engineers to stand up and speak out on behalf of our profession and I pledged to lead from the front. Second, I emphasised the importance of building bridges with other stakeholders on the chemical engineering landscape and I promised to build on Chemical Engineering Matters as a platform for communication, both inside and outside IChemE. Finally, I stressed the need for the Institution to be more active in emphasising to those outside our profession, not least governments, the role of chemical engineering in securing sustainable energy solutions. There have been developments on all fronts. In May, I initiated a daily blog with the intention of shining a light on chemical engineering on all 365 days of my presidency. My chemical engineering good news stories have been read and shared by more than 100,000 readers in 160 countries. I trust that this has provided inspiration and encouragement to chemical engineers everywhere. The partnerships with the South African Institution of Chemical Engineers and the Mary Kay O’Connor Process Safety Center evidence our willingness to collaborate; as does the joint statement on climate change that we signed with the Royal Society of Chemistry in November. Energy and climate change issues constantly feature as the top priority for our members. -

High Level Framework for Process Safety Management
High level framework for process safety management IMPORTANT: This file is subject to a licence agreement issued by the Energy Institute, London, UK. All rights reserved. It may only be used in accordance with the licence terms and conditions. It must not be forwarded to, or stored or accessed by, any unauthorised user. Enquiries: e: [email protected] t: +44 (0)207 467 7100 HIGH LEVEL FRAMEWORK FOR PROCESS SAFETY MANAGEMENT 1st edition December 2010 Published by ENERGY INSTITUTE, LONDON The Energy Institute is a professional membership body incorporated by Royal Charter 2003 Registered charity number 1097899 IMPORTANT: This file is subject to a licence agreement issued by the Energy Institute, London, UK. All rights reserved. It may only be used in accordance with the licence terms and conditions. It must not be forwarded to, or stored or accessed by, any unauthorised user. Enquiries: e: [email protected] t: +44 (0)207 467 7100 The Energy Institute (EI) is the leading chartered professional membership body supporting individuals and organisations across the energy industry. With a combined membership of over 13 500 individuals and 300 companies in 100 countries, it provides an independent focal point for the energy community and a powerful voice to engage business and industry, government, academia and the public internationally. As a Royal Charter organisation, the EI offers professional recognition and sustains personal career development through the accreditation and delivery of training courses, conferences and publications and networking opportunities. It also runs a highly valued technical work programme, comprising original independent research and investigations, and the provision of EI technical publications to provide the international industry with information and guidance on key current and future issues. -

Good Plant Design and Operation for Onshore Carbon Capture Installations and Onshore Pipelines
Good plant design and operation for onshore carbon capture installations and onshore pipelines Registered Charity Number 1097899 GOOD PLANT DESIGN AND OPERATION FOR ONSHORE CARBON CAPTURE INSTALLATIONS AND ONSHORE PIPELINES A Recommended Practice Guidance Document First edition September 2010 Published by ENERGY INSTITUTE, LONDON The Energy Institute is a professional membership body incorporated by Royal Charter 2003 Registered charity number 1097899 The Energy Institute (EI) is the leading chartered professional membership body supporting individuals and organisations across the energy industry. With a combined membership of over 13 500 individuals and 300 companies in 100 countries, it provides an independent focal point for the energy community and a powerful voice to engage business and industry, government, academia and the public internationally. As a Royal Charter organisation, the EI offers professional recognition and sustains personal career development through the accreditation and delivery of training courses, conferences and publications and networking opportunities. It also runs a highly valued technical work programme, comprising original independent research and investigations, and the provision of EI technical publications to provide the international industry with information and guidance on key current and future issues. The EI promotes the safe, environmentally responsible and efficient supply and use of energy in all its forms and applications. In fulfilling this purpose the EI addresses the depth and breadth of energy and the energy system, from upstream and downstream hydrocarbons and other primary fuels and renewables, to power generation, transmission and distribution to sustainable development, demand side management and energy efficiency. Offering learning and networking opportunities to support career development, the EI provides a home to all those working in energy, and a scientific and technical reservoir of knowledge for industry. -

The DA GHGI Improvement Programme 2009-2010 Industry Sector Task
The DA GHGI Improvement Programme 2009-2010 Industry Sector Task DECC, The Scottish Government, The Welsh Assembly Government and the Northern Ireland Department of the Environment AEAT/ENV/R/2990_3 Issue 1 May 2010 DA GHGI Improvements 2009-2010: Industry Task Restricted – Commercial AEAT/ENV/R/2990_3 Title The DA GHGI Improvement Programme 2009-2010: Industry Sector Task Customer DECC, The Scottish Government, The Welsh Assembly Government and the Northern Ireland Department of the Environment Customer reference NAEI Framework Agreement/DA GHGI Improvement Programme Confidentiality, Crown Copyright copyright and reproduction File reference 45322/2008/CD6774/GT Reference number AEAT/ENV/R/2990_3 /Issue 1 AEA Group 329 Harwell Didcot Oxfordshire OX11 0QJ Tel.: 0870 190 6584 AEA is a business name of AEA Technology plc AEA is certificated to ISO9001 and ISO14001 Authors Name Stuart Sneddon and Glen Thistlethwaite Approved by Name Neil Passant Signature Date 20th May 2010 ii AEA Restricted – Commercial DA GHGI Improvements 2009-2010: Industry Task AEAT/ENV/R/2990_3 Executive Summary This research has been commissioned under the UK and DA GHG inventory improvement programme, and aims to research emissions data for a group of source sectors and specific sites where uncertainties have been identified in the scope and accuracy of available source data. Primarily this research aims to review site-specific data and regulatory information, to resolve differences between GHG data reported across different emission reporting mechanisms. The research has comprised: 1) Data review from different reporting mechanisms (IPPC, EU ETS and EEMS) to identify priority sites (primarily oil & gas terminals, refineries and petrochemicals), i.e. -

Jet Fuel Thermal Oxidation Tube Rating Training Guidelines (IP 323)
Jet fuel thermal oxidation tube rating training guidelines (IP 323) This publication has been produced as a result of work carried out within the Technical Team of the Energy Institute (EI), funded by the EI’s Technical Partners. The EI’s Technical Work Programme provides industry with cost-effective, value-adding knowledge on key current and future issues affecting those operating in the energy sector, both in the UK and internationally. For further information, please visit http://www.energyinst.org The EI gratefully acknowledges the financial contributions towards the scientific and technical programme from the following companies BG Group Murco Petroleum Ltd BP Exploration Operating Co Ltd Nexen BP Oil UK Ltd Premier Oil Centrica RWE npower Chevron Saudi Aramco ConocoPhillips Ltd Shell UK Oil Products Limited EDF Energy Shell U.K. Exploration and Production Ltd ENI Statoil Hydro E. ON UK Talisman Energy (UK) Ltd ExxonMobil International Ltd Total E&P UK plc Kuwait Petroleum International Ltd Total UK Limited Maersk Oil North Sea UK Limited World Fuel Services Copyright © 2011 by the Energy Institute, London. The Energy Institute is a professional membership body incorporated by Royal Charter 2003. Registered charity number 1097899, England All rights reserved No part of this book may be reproduced by any means, or transmitted or translated into a machine language without the written permission of the publisher. ISBN 978 0 85293 561 3 Published by the Energy Institute The information contained in this publication is provided as guidance only and while every reasonable care has been taken to ensure the accuracy of its contents, the Energy Institute cannot accept any responsibility for any action taken, or not taken, on the basis of this information. -

Modified UK National Implementation Measures for Phase III of the EU Emissions Trading System
Modified UK National Implementation Measures for Phase III of the EU Emissions Trading System As submitted to the European Commission in April 2012 following the first stage of their scrutiny process This document has been issued by the Department of Energy and Climate Change, together with the Devolved Administrations for Northern Ireland, Scotland and Wales. April 2012 UK’s National Implementation Measures submission – April 2012 Modified UK National Implementation Measures for Phase III of the EU Emissions Trading System As submitted to the European Commission in April 2012 following the first stage of their scrutiny process On 12 December 2011, the UK submitted to the European Commission the UK’s National Implementation Measures (NIMs), containing the preliminary levels of free allocation of allowances to installations under Phase III of the EU Emissions Trading System (2013-2020), in accordance with Article 11 of the revised ETS Directive (2009/29/EC). In response to queries raised by the European Commission during the first stage of their assessment of the UK’s NIMs, the UK has made a small number of modifications to its NIMs. This includes the introduction of preliminary levels of free allocation for four additional installations and amendments to the preliminary free allocation levels of seven installations that were included in the original NIMs submission. The operators of the installations affected have been informed directly of these changes. The allocations are not final at this stage as the Commission’s NIMs scrutiny process is ongoing. Only when all installation-level allocations for an EU Member State have been approved will that Member State’s NIMs and the preliminary levels of allocation be accepted. -

Download Original Attachment
Operator Name Location Name Address Name Address Street Address Town Address County Address Postcode 1 Address Postcode 2 Incumbent Duty Type Text Previous Name LA Code Local Authority Country AMG Superalloys UK Limited Rotherham Fullerton Road Rotherham South Yorkshire S60 1DL COMAH Upper Tier Operator (was London & Scandinavian Metallurgical Co Ltd) 4415 Rotherham England Anglian Water Services Limited Wing Water Treatment Works Morcott Road Oakham Rutland LE15 8SA COMAH Upper Tier Operator 2470 Rutland UA England Arch Timber Protection Limited Huddersfield Huddersfield Works Leeds Road Huddersfield West Yorkshire HD2 1YU COMAH Upper Tier Operator (was Arch UK Biocides Ltd) 4715 Kirklees England Argenta Dundee Limited Dundee Dunsinane Industrial Estate Kinnoull Road Dundee Angus DD2 3XR COMAH Upper Tier Operator (was Vericore Limited) 9059 Dundee UA Scotland Associated British Ports Immingham Dock Immingham Dock Immingham Lincolnshire DN40 2NS COMAH Upper Tier Operator 2002 North East Lincolnshire England Associated Petroleum Terminals (Immingham) Limited Immingham Main Terminal Queens Road Immingham North East Lincolnshire DN40 2PN COMAH Upper Tier Operator 2002 North East Lincolnshire England Avanti Gas Limited Ellesmere Port Britannia Road Ellesmere Port Cheshire CH65 4HB COMAH Upper Tier Operator (was Shell Gas Limited) 4325 Wirral England Avara Avlon Pharma Services Limited Avlon Works Severn Road Bristol South Gloucestershire BS10 7ZE COMAH Upper Tier Operator (was AstraZeneca UK Limited) 0119 South Gloucs UA England BAE Systems -

Design, Construction, Modification, Maintenance and Decommissioning of Filling Stations
Design, construction, modification, maintenance and decommissioning of filling stations 3rd edition Jointly published by the APEA and Energy Institute 3rd edition APEA/EI DESIGN, CONSTRUCTION, MODIFICATION, MAINTENANCE AND DECOMMISSIONING OF FILLING STATIONS 3rd edition June 2011 Published by The Association for Petroleum and Explosives Administration (APEA) and ENERGY INSTITUTE, LONDON The Energy Institute is a professional membership body incorporated by Royal Charter 2003 Registered charity number 1097899 The Energy Institute (EI) is the leading chartered professional membership body supporting individuals and organisations across the energy industry. With a combined membership of over 14 000 individuals and 300 companies in 100 countries, it provides an independent focal point for the energy community and a powerful voice to engage business and industry, government, academia and the public internationally. As a Royal Charter organisation, the EI offers professional recognition and sustains personal career development through the accreditation and delivery of training courses, conferences and publications and networking opportunities. It also runs a highly valued technical work programme, comprising original independent research and investigations, and the provision of EI technical publications to provide the international industry with information and guidance on key current and future issues. For further information, please visit http://www.energyinst.org The EI gratefully acknowledges the financial contributions towards the scientific and technical programme from the following companies: BG Group Murco Petroleum Ltd BP Exploration Operating Co Ltd Nexen BP Oil UK Ltd Premier Oil Centrica RWE npower Chevron Saudi Aramco ConocoPhillips Ltd Shell UK Oil Products Limited EDF Energy Shell U.K. Exploration and Production Ltd ENI Statoil Hydro E. -

PIPE SUPPORTS LTD Company Profile
PIPE SUPPORTS LTD Company Profile CONTENTS Page 2. Address, Location and Function. Page 4. Financial, Legal and Commercial. Page 5. Facilities. Page 7. Design Capability. Page 8. Quality Assurance and Controls. Page 8. Membership of Trade Associations and Technical Committees. Page 8. Sales Representation. 21/01/2014 Page 1 of 8 Address, Location and Function 1.1 Name and Address: Pipe Supports Limited Unit 22, West Stone Berry Hill Industrial Estate Droitwich Worcs WR9 9AS 1.2 Telephone Number: +44 (0) 1905 795500 1.3 Fax Number: +44 (0) 1905 794126 1.4 E-mail address: [email protected] 1.5 Web site address: www.PipeSupports.com 1.6 Factory Address: Unit 22, West Stone Berry Hill Industrial Estate Droitwich Worcs WR9 9AS 1.7 Location: Approximately 2.5 miles from M5 Junction 5, and 6 miles from M5 Junction 6, just off A38. 1.8 Access to Factory: Good via driveway - minimum width approximately 5m. Roller shutter opening into factory 4.5 m x 4.5 m. EOT crane capacity of 5 tonnes. Max. lift height 4 metres. 3 Fork lift trucks capacity 5 tonnes. 1.9 Principal Function of Company: The design, manufacture and supply of pipe hangers, restraints and associated equipment for all types of pipework installations in the industrial, commercial, power generation and petrochemical sectors. Secondary services include site survey and piping analysis combined with the long-term monitoring of piping performance. 1.10 Product Listing: PSDesigner – Software for Designing Pipe hanger Assemblies. PSLCAD - Computerised Support Design / Drawing System -

API/EI 1585 Guidance in the Cleaning of Aviation Fuel Hydrant Systems At
API/EI 1585 Guidance in the cleaning of aviation fuel hydrant systems at airports 2nd edition Information about API Publications, Programs and Services is available on the World Wide Web at: www.api.org 1220 L Street Northwest Washington, D.C. 20005-4070 202-682-8000 API/EI 1585 Energy Institute This publication has been produced as a result of 61 New Cavendish Street work carried out within the Technical Team of the Guidance in the cleaning of London W1G 7AR, UK Energy Institute (EI), funded by the EI’s Technical aviation fuel hydrant systems at airports Partners. The EI’s Technical Work Programme t: +44 (0) 20 7467 7157 provides industry with cost effective, value adding f: +44 (0) 20 7255 1472 knowledge on key current and future issues e: [email protected] affecting those operating in the energy sector, www.energyinst.org.uk both in the UK and beyond. ISBN 978 0 85293 490 6 2nd edition Registered Charity Number 1097899 GUIDANCE IN THE CLEANING OF AVIATION FUEL HYDRANT SYSTEMS AT AIRPORTS API/EI 1585 Second edition November 2007 GUIDANCE IN THE CLEANING OF AVIATION FUEL HYDRANT SYSTEMS AT AIRPORTS API/EI 1585 Second edition November 2007 Published jointly by API and ENERGY INSTITUTE, LONDON The Energy Institute is a professional membership body incorporated by Royal Charter 2003 Registered charity number 1097899 The Energy Institute gratefully acknowledges the financial contributions towards the scientific and technical programme from the following companies: BG Group Murco Petroleum Ltd BHP Billiton Limited Nexen BP Exploration Operating Co Ltd Saudi Aramco BP Oil UK Ltd Shell UK Oil Products Limited Chevron Shell U.K. -
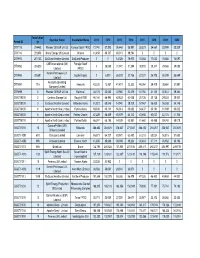
Permit ID Installation ID Operator Name Installation Name 2013 2014
Installation Operator Name Installation Name 2013 2014 2015 2016 2017 2018 2019 2020 Permit ID ID DTI1015 204463 Premier Oil E&P UK Ltd Voyager Spirit FPSO 72,740 37,302 36,642 35,981 35,321 34,660 33,999 33,339 DTI1016 202656 Ithaca Energy UK Limited Athena 41,458 40,737 40,015 39,294 0000 DTI9910 207103 EnQuest Heather Limited EnQuest Producer 0 0 14,726 79,970 78,502 77,033 75,565 74,097 CNR International (UK) Petrojarl Banff DTI9950 206523 0 26,039 31,970 31,394 30,818 30,241 29,665 29,088 Limited FPSO Nexen Petroleum U.K. DTI9960 206591 Golden Eagle 0 6,010 26,378 27,736 27,227 26,718 26,209 25,699 Limited Anasuria Operating DTI9991 715 Anasuria 43,325 13,457 41,811 22,330 40,264 39,479 38,684 37,887 Company Limited DTI9999 8 Premier Oil E&P UK Ltd Balmoral 34,174 33,580 32,980 32,379 31,760 31,140 30,513 29,884 GB-DTI0200 2 Centrica Storage Ltd Rough 47/3B 66,144 64,995 63,833 62,658 30,736 30,136 29,530 28,921 GB-DTI0300 3 EnQuest Heather Limited Kittiwake Alpha 61,921 60,846 14,940 29,329 57,547 56,425 55,288 54,148 GB-DTI0400 4 Apache North Sea Limited Forties Alpha 69,336 68,131 66,913 65,682 64,437 63,181 61,909 60,632 GB-DTI0600 6 Apache North Sea Limited Forties Charlie 47,229 46,409 45,579 44,740 43,892 43,037 42,170 41,300 GB-DTI0700 7 Apache North Sea Limited Forties Delta 66,347 65,195 64,029 62,851 61,660 60,458 59,240 58,019 ConocoPhillips (UK) GB-DTI1000 10 Britannia 286,653 281,674 276,637 271,547 266,402 261,207 255,947 250,670 Britannia Limited GB-DTI-1001 693 Chrysaor Limited Lomond 65,871 64,727 63,570 62,400 61,218 60,024 58,815 57,603 GB-DTI-1002 696 Chrysaor Limited Everest North 41,385 40,666 39,939 39,204 38,461 37,711 36,952 36,190 GB-DTI1003 686 Britoil Ltd Bruce 142,795 280,628 137,805 270,538 265,413 260,237 254,997 249,739 Spirit Energy North Sea Oil Sevan Marine GB-DTI-1010 1123 127,129 124,921 122,687 120,430 118,148 115,844 113,512 111,171 Limited Hummingbird GB-DTI1011 18 Perenco UK Limited Thames Alpha 23,338 22,933 0 0 0000 Nexen Petroleum U.K. -

The United Kingdom 2002 Review INTERNATIONAL ENERGY AGENCY
INTERNATIONAL ENERGY AGENCY Energy Policies of IEA Countries The United Kingdom 2002 Review INTERNATIONAL ENERGY AGENCY Energy Policies of IEA Countries The United Kingdom 2002 Review INTERNATIONAL ENERGY AGENCY ORGANISATION FOR 9, rue de la Fédération, ECONOMIC CO-OPERATION 75739 Paris, cedex 15, France AND DEVELOPMENT The International Energy Agency (IEA) is an Pursuant to Article 1 of the Convention signed in Paris autonomous body which was established in November on 14th December 1960, and which came into force 1974 within the framework of the Organisation for on 30th September 1961, the Organisation for Economic Co-operation and Development (OECD) to Economic Co-operation and Development (OECD) shall implement an international energy programme. promote policies designed: It carries out a comprehensive programme of energy co- • to achieve the highest sustainable economic growth operation among twenty-six* of the OECD’s thirty and employment and a rising standard of living in Member countries. The basic aims of the IEA are: Member countries, while maintaining financial stability, and thus to contribute to the development • to maintain and improve systems for coping with oil of the world economy; supply disruptions; • to contribute to sound economic expansion in • to promote rational energy policies in a global Member as well as non-member countries in the context through co-operative relations with non- process of economic development; and member countries, industry and international organisations; • to contribute to the expansion