11-Major Metabolic Pathway TEAM436
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oxidative Pentose Phosphate Pathway Enzyme 6-Phosphogluconate Dehydrogenase Plays a Key Role in Breast Cancer Metabolism
biology Article Oxidative Pentose Phosphate Pathway Enzyme 6-Phosphogluconate Dehydrogenase Plays a Key Role in Breast Cancer Metabolism Ibrahim H. Polat 1,2,3 ,Míriam Tarrado-Castellarnau 1,4 , Rohit Bharat 1, Jordi Perarnau 1 , Adrian Benito 1,5 , Roldán Cortés 1, Philippe Sabatier 2 and Marta Cascante 1,4,* 1 Department of Biochemistry and Molecular Biomedicine and Institute of Biomedicine (IBUB), Faculty of Biology, Universitat de Barcelona, Av Diagonal 643, 08028 Barcelona, Spain; [email protected] (I.H.P.); [email protected] (M.T.-C.); [email protected] (R.B.); [email protected] (J.P.); [email protected] (A.B.); [email protected] (R.C.) 2 Equipe Environnement et Prédiction de la Santé des Populations, Laboratoire TIMC (UMR 5525), CHU de Grenoble, Université Grenoble Alpes, 38700 CEDEX La Tronche, France; [email protected] 3 Department of Medicine, Hematology/Oncology, Goethe-University Frankfurt, 60590 Frankfurt, Germany 4 Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Instituto de Salud Carlos III (ISCIII), 28001 Madrid, Spain 5 Division of Cancer, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London W12 0NN, UK * Correspondence: [email protected] Simple Summary: Cancer cells alter their metabolism to maintain their high need for energy, produce Citation: Polat, I.H.; enough macromolecules for biosynthesis, and preserve their redox status. The investigation of Tarrado-Castellarnau, M.; Bharat, R.; cancer cell-specific metabolic alterations has vital importance to identify targets to be exploited for Perarnau, J.; Benito, A.; Cortés, R.; therapeutic development. The pentose phosphate pathway (PPP) is often highly activated in tumor Sabatier, P.; Cascante, M. -

A New Insight Into Role of Phosphoketolase Pathway in Synechocystis Sp
www.nature.com/scientificreports OPEN A new insight into role of phosphoketolase pathway in Synechocystis sp. PCC 6803 Anushree Bachhar & Jiri Jablonsky* Phosphoketolase (PKET) pathway is predominant in cyanobacteria (around 98%) but current opinion is that it is virtually inactive under autotrophic ambient CO2 condition (AC-auto). This creates an evolutionary paradox due to the existence of PKET pathway in obligatory photoautotrophs. We aim to answer the paradox with the aid of bioinformatic analysis along with metabolic, transcriptomic, fuxomic and mutant data integrated into a multi-level kinetic model. We discussed the problems linked to neglected isozyme, pket2 (sll0529) and inconsistencies towards the explanation of residual fux via PKET pathway in the case of silenced pket1 (slr0453) in Synechocystis sp. PCC 6803. Our in silico analysis showed: (1) 17% fux reduction via RuBisCO for Δpket1 under AC-auto, (2) 11.2–14.3% growth decrease for Δpket2 in turbulent AC-auto, and (3) fux via PKET pathway reaching up to 252% of the fux via phosphoglycerate mutase under AC-auto. All results imply that PKET pathway plays a crucial role under AC-auto by mitigating the decarboxylation occurring in OPP pathway and conversion of pyruvate to acetyl CoA linked to EMP glycolysis under the carbon scarce environment. Finally, our model predicted that PKETs have low afnity to S7P as a substrate. Metabolic engineering of cyanobacteria provides many options for producing valuable compounds, e.g., acetone from Synechococcus elongatus PCC 79421 and butanol from Synechocystis sp. strain PCC 68032. However, certain metabolites or overproduction of intermediates can be lethal. Tere is also a possibility that required mutation(s) might be unstable or the target bacterium may even be able to maintain the fux distribution for optimal growth balance due to redundancies in the metabolic network, such as alternative pathways. -

Enzyme Characterisation and Kinetic Modelling of the Pentose Phosphate
1 Enzyme characterisation and kinetic modelling of the pentose 2 phosphate pathway in yeast 1;2 3 Hanan L. Messiha Edward Kent 1;3;4 Naglis Malys 1;2;6 Kathleen M. Carroll 1;5 Neil Swainston 1;4 s 1;4;7;∗ t Pedro Mendes 1;4 n Kieran Smallbone i r P 1Manchester Centre for Integrative Systems Biology e r 2Faculty of Life Sciences 3 P Doctoral Training Centre in Integrative Systems Biology 4School of Computer Science 5School of Chemistry University of Manchester, M13 9PL, UK. 6School of Life Sciences, Gibbet Hill Campus, University of Warwick, Coventry, UK. 7Center for Quantitative Medicine and Department of Cell Biology, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030, USA. 4 Abstract 5 We present the quantification and kinetic characterisation of the enzymes of the pentose 6 phosphate pathway in Saccharomyces cerevisiae. The data are combined into a mathematical 7 model that describes the dynamics of this system and allows us to predict changes in metabo- 8 lite concentrations and fluxes in response to perturbations. We use the model to study the 9 response of yeast to a glucose pulse. We then combine the model with an existing glycolysis 10 model to study the effect of oxidative stress on carbohydrate metabolism. The combina- 11 tion of these two models was made possible by the standardised enzyme kinetic experiments 12 carried out in both studies. This work demonstrates the feasibility of constructing larger 13 network-scale models by merging smaller pathway-scale models. ∗To whom correspondence should be addressed at [email protected] PeerJ PrePrints | http://dx.doi.org/10.7287/peerj.preprints.146v4 | CC-BY 3.0 Open Access | received: 10 Apr 2014, published: 10 Apr 2014 1 14 Introduction 15 The pentose phosphate pathway (PPP) is a central and widely conserved metabolic pathway of car- 16 bohydrate metabolism which, in eukaryotic cells, is located in the cytoplasm (see Figure 1). -

Lecture 7 - the Calvin Cycle and the Pentose Phosphate Pathway
Lecture 7 - The Calvin Cycle and the Pentose Phosphate Pathway Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction The Calvin cycle Text The dark reactions of photosynthesis in green plants Reduces carbon from CO2 to hexose (C6H12O6) Requires ATP for free energy and NADPH as a reducing agent. 2 2 Introduction NADH versus Text NADPH 3 3 Introduction The Pentose Phosphate Pathway Used in all organisms Glucose is oxidized and decarboxylated to produce reduced NADPH Used for the synthesis and degradation of pentoses Shares reactions with the Calvin cycle 4 4 1. The Calvin Cycle Source of carbon is CO2 Text Takes place in the stroma of the chloroplasts Comprises three stages Fixation of CO2 by ribulose 1,5-bisphosphate to form two 3-phosphoglycerate molecules Reduction of 3-phosphoglycerate to produce hexose sugars Regeneration of ribulose 1,5-bisphosphate 5 5 1. Calvin Cycle Three stages 6 6 1.1 Stage I: Fixation Incorporation of CO2 into 3-phosphoglycerate 7 7 1.1 Stage I: Fixation Rubisco: Ribulose 1,5- bisphosphate carboxylase/ oxygenase 8 8 1.1 Stage I: Fixation Active site contains a divalent metal ion 9 9 1.2 Rubisco Oxygenase Activity Rubisco also catalyzes a wasteful oxygenase reaction: 10 10 1.3 State II: Formation of Hexoses Reactions similar to those of gluconeogenesis But they take place in the chloroplasts And use NADPH instead of NADH 11 11 1.3 State III: Regeneration of Ribulose 1,5-Bisphosphosphate Involves a sequence of transketolase and aldolase reactions. 12 12 1.3 State III: -

Sterile Spikelets Assimilate Carbon in Sorghum and Related Grasses
bioRxiv preprint doi: https://doi.org/10.1101/396473; this version posted January 5, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. It is not subject to copyright under 17 USC 105 and is also made available for use under a CC0 license. 1 Sterile spikelets assimilate carbon in sorghum and related grasses Taylor AuBuchon-Elder1,a, Viktoriya Coneva1,a,d, David M. Goad a,b, Doug K. Allen2,a,c,e, and Elizabeth A. Kellogg2,a,e aDonald Danforth Plant Science Center, St. Louis, Missouri, 63132 USA bDepartment of Biology, Washington University, St. Louis, Missouri, 63130 USA cUSDA-ARS, St. Louis, Missouri, 63132 USA 1These authors contributed equally to this work. 2These authors contributed equally to this work. dCurrent address: Kenyon College, Gambier, OH 43022 eAddress correspondence to: [email protected]; [email protected] ORCID IDs: 0000-0002-0640-5135 (V.C.); 0000-0001-8658-6660 (D.M.G.); 0000-0001-8599- 8946 (D.K.A.); 0000-0003-1671-7447 (E.A.K.) Short title: Carbon assimilation in sorghum spikelets The author responsible for distribution of materials integral to the findings presented in this article is Elizabeth A. Kellogg ([email protected]). bioRxiv preprint doi: https://doi.org/10.1101/396473; this version posted January 5, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. It is not subject to copyright under 17 USC 105 and is also made available for use under a CC0 license. -

The Pentose Phosphate Pathway and Its Involvement in Cisplatin Resistance
International Journal of Molecular Sciences Review The Pentose Phosphate Pathway and Its Involvement in Cisplatin Resistance Isabella Giacomini 1, Eugenio Ragazzi 1 , Gianfranco Pasut 2 and Monica Montopoli 1,3,* 1 Department of Pharmaceutical and Pharmacological Sciences, University of Padua, Largo Egidio Meneghetti 2, 35131 Padova, Italy; [email protected] (I.G.); [email protected] (E.R.) 2 Department of Pharmaceutical and Pharmacological Sciences, University of Padua, Via Marzolo 5, 35131 Padova, Italy; [email protected] 3 Veneto Institute of Molecular Medicine, Via Giuseppe Orus 2, 35129 Padova, Italy * Correspondence: [email protected]; Tel.: +39-049-827-5090 Received: 30 December 2019; Accepted: 29 January 2020; Published: 31 January 2020 Abstract: Cisplatin is the first-line treatment for different types of solid tumors, such as ovarian, testicular, bladder, cervical, head and neck, lung, and esophageal cancers. The main problem related to its clinical use is the onset of drug resistance. In the last decades, among the studied molecular mechanisms of cisplatin resistance, metabolic reprogramming has emerged as a possible one. This review focuses on the pentose phosphate pathway (PPP) playing a pivotal role in maintaining the high cell proliferation rate and representing an advantage for cancer cells. In particular, the oxidative branch of PPP plays a role in oxidative stress and seems to be involved in cisplatin resistance. In light of these considerations, it has been demonstrated that overexpression and higher enzymatic activity of different enzymes of both oxidative and non-oxidative branches (such as glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and transketolase) increase cisplatin resistance, and their silencing or combined treatment with cisplatin could restore cisplatin sensitivity. -

RESPIRATION Pentose Phosphate Pathway Or Hexose Monophosphate Pathway
RESPIRATION Pentose Phosphate Pathway or Hexose Monophosphate Pathway Pentose phosphate pathway or hexose monophosphate pathway (HMP pathway) is the other common pathway to break down glucose to pyruvate and operates in both aerobic and anaerobic conditions. This pathway produces NADPH, which carries chemical energy in the form of reducing power and is used almost universally as the reductant in anabolic (energy utilization) pathways (e.g., fatty acid biosynthesis, cholesterol biosynthesis, nucleotide biosynthesis) and detoxification pathways (e.g., reduction of oxidized glutathione, cytochrome P450 monooxygenases). Also, the pentose phosphate pathway generates pentose sugar ribose and its derivatives, which are necessary for the biosynthesis of nucleic acids (DNA and RNA) as well as ATP, NADH, FAD, and coenzyme A. In this way, though the pentose phosphate pathway may be a source of energy in many microorganisms, it is more often of greater importance in various biosynthetic pathways. Pentose phosphate pathway consists of two phases: the oxidative phase and the non-oxidative phase. Oxidative Phase: The oxidative phase of the pentose phosphate pathway initiates with the conversion of glucose 6- phosphate to 6-Phosphogluconate. NADP+ is the electron acceptor yielding NADPH during this reaction. 6-Phosphogluconate, a six-carbon sugar, is then oxidativelydecarboxylated to yield ribulose 5-phosphate, a five-carbon sugar. NADP+ is again the electron acceptor yielding NADPH. In the final step of oxidative phase, there is isomerisation of ribulose 5-phosphatc to ribose 5- phosphate by phosphopentose isomerase and the conversion of ribulose 5-phosphate into its epimerxylulose 5-phosphate by phosphopentose epimerase for the transketolase reaction in non- oxidative phase. -

Chemoenzymatic Synthesis of Novel, Structurally Diverse Compounds
Chemoenzymatic synthesis of novel, structurally diverse compounds A Thesis Submitted for the Degree of Doctor of Philosophy to the University of London Lydia G Coward Department of Biochemical Engineering University College London 1 I, Lydia Grace Coward confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 For my fantastic family. 3 Abstract The use of Diels Alder cycloaddition chemistries to access a diverse range of useful cyclic structures is well established throughout literature. However, the value of the product may be enhanced further still by linking this reaction with subsequent (biocatalytic) steps to create novel, structurally demanding, optically pure compounds. This project investigates the linking of Diels Alder (DA) chemistry to the enzyme, transketolase (TK) as a model integration pathway of a chemical syntheses and a biological transformation. The two-step process aims to provide a framework to synthesise small structurally diverse compounds with high enantiomeric excess. The demand for optically pure compounds is becoming a necessity due to the adverse affects frequently introduced by racemic compounds and the cost implications of the material possessing often only 50% active compound. Recombinant wild type Eschericha coli transketolase (EC 2.2.1.1) (WT-TK) was overexpressed in E. coli for the biocatalytic step of this two step synthesis. A substrate walking approach whereby a range of sequentially linked cyclic aldehydes, were applied to wild type transketolase and potential activity detected. Transketolase mutants, previously constructed based on information derived from the structural position within the active site of the dimeric enzyme were subsequently screened for activity with the cycloadduct of the Diels Alder reaction as aldehyde acceptor substrate for TK. -

PENTOSE PHOSPHATE PATHWAY — Restricted for Students Enrolled in MCB102, UC Berkeley, Spring 2008 ONLY
Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Bryan Krantz: University of California, Berkeley MCB 102, Spring 2008, Metabolism Lecture 5 Reading: Ch. 14 of Principles of Biochemistry, “Glycolysis, Gluconeogenesis, & Pentose Phosphate Pathway.” PENTOSE PHOSPHATE PATHWAY This pathway produces ribose from glucose, and it also generates 2 NADPH. Two Phases: [1] Oxidative Phase & [2] Non-oxidative Phase + + Glucose 6-Phosphate + 2 NADP + H2O Ribose 5-Phosphate + 2 NADPH + CO2 + 2H ● What are pentoses? Why do we need them? ◦ DNA & RNA ◦ Cofactors in enzymes ● Where do we get them? Diet and from glucose (and other sugars) via the Pentose Phosphate Pathway. ● Is the Pentose Phosphate Pathway just about making ribose sugars from glucose? (1) Important for biosynthetic pathways using NADPH, and (2) a high cytosolic reducing potential from NADPH is sometimes required to advert oxidative damage by radicals, e.g., ● - ● O2 and H—O Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Two Phases of the Pentose Pathway Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY NADPH vs. NADH Metabolism Lecture 5 — PENTOSE PHOSPHATE PATHWAY — Restricted for students enrolled in MCB102, UC Berkeley, Spring 2008 ONLY Oxidative Phase: Glucose-6-P Ribose-5-P Glucose 6-phosphate dehydrogenase. First enzymatic step in oxidative phase, converting NADP+ to NADPH. Glucose 6-phosphate + NADP+ 6-Phosphoglucono-δ-lactone + NADPH + H+ Mechanism. Oxidation reaction of C1 position. Hydride transfer to the NADP+, forming a lactone, which is an intra-molecular ester. -

Chapter 23 Gluconeogenesis Gluconeogenesis, Con't
BCH 4054 Spring 2001 Chapter 23 Lecture Notes Slide 1 Chapter 23 Gluconeogenesis Glycogen Metabolism Pentose Phosphate Pathway Slide 2 Gluconeogenesis • Humans use about 160 g of glucose per day, about 75% for the brain. • Body fluids and glycogen stores supply only a little over a day’s supply. • In absence of dietary carbohydrate, the needed glucose must be made from non- carbohydrate precursors. • That process is called gluconeogenesis. Slide 3 Gluconeogenesis, con’t. • Brain and muscle consume most of the glucose. • Liver and kidney are the main sites of gluconeogenesis. • Substrates include pyruvate, lactate, glycerol, most amino acids, and all TCA intermediates. • Fatty acids cannot be converted to glucose in animals. • (They can in plants because of the glyoxalate cycle.) Chapter 23, page 1 Slide 4 Remember it is necessary for the pathways to differ in some respects, Gluconeogenesis, con’t. so that the overall DG can be negative in each direction. Usually • Substrates include anything that can be converted the steps with large negative DG of to phosphoenolpyruvate . one pathway are replaced in the • Many of the reactions are the same as those in reverse pathway with reactions that glycolysis. have a large negative DG in the • All glycolytic reactions which are near equilibrium can opposite direction. operate in both directions. • The three glycolytic reactions far from equilibrium (large -DG) must be bypassed. • A side by side comparison is shown in Fig 23.1. Slide 5 Unique Reactions of Gluconeogenesis • Recall that pyruvate kinase, though named in reverse, is not reversible and has a DG of –23 kJ/mol. -

Deliverable 1.5 Model of Plant Photosynthesis
PROJECT ACRONYM: FutureAgriculture PROJECT TITLE: Transforming the future of agriculture through synthetic photorespiration Deliverable 1.5 Model of plant photosynthesis TOPIC H2020-FETOPEN-2014-2015-RIA GA 686330 Arren Bar-Even CONTACT PERSON [email protected] NATURE Report (R) DISSEMINATION LEVEL PU DUE DATE 31/12/2018 ACTUAL DELIVERED DATE 14/12/2018 AUTHOR Christian Edlich-Muth This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 686330. Table of Revisions REVISION NUMBER DATE WORK PERFORMED CONTRIBUTOR(S) 1 05/12/2018 Document preparation MPIMP 2 06/12/2018 Formatting IN 3 13/12/2018 Revision Consortium D1.5 – Model of plant photosynthesis Page 2 03/04/2018 Contents 1ExecutiveSummary 5 2Cooperationbetweenparticipants 6 3 Core report 7 3.1 Theory............................................. 7 3.1.1 Pathways . 7 3.1.2 The stoichiometric consumer model of photosynthesis . 9 3.1.3 Stoichiometric-kineticmodel. 13 3.1.4 Physicochemicalparameters. 24 3.2 Results............................................. 27 3.2.1 Parametersandvariables .............................. 27 3.2.2 Rubiscokinetics ................................... 28 3.2.3 Limitation by light . 30 3.2.4 LimitationbyenzymeactivitiesotherthanRubisco. 31 3.2.5 Limitation by a Calvin cycle enzyme . 31 3.2.6 Limitationbyaphotorespiratoryenzyme . 34 3.2.7 Limitationbyaphotorespirationcarboxylase . 35 4 Conclusions 37 D1.5Modelofplantphotosynthesis Page3 03/04/2018 Table of Charts 1 Bypass coefficients ........................................ 11 2 Flux distribtion of the Calvin cycle and photorespiratory bypasses . 20 3 Kinetic constants of Rubisco and carbon dioxide diffusion .................. 28 4 Calvin cycle enzyme total activities . 33 Table of Figures 1 Energy requirements of native photorespiration and the Calvin cycle . -
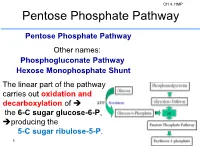
Pentose Phosphate Pathway
CH 4. HMP Pentose Phosphate Pathway Pentose Phosphate Pathway Other names: Phosphogluconate Pathway Hexose Monophosphate Shunt The linear part of the pathway carries out oxidation and decarboxylation of the 6-C sugar glucose-6-P, producing the 5-C sugar ribulose-5-P. 1 2 CH 4. HMP Glucose metabolism via the oxidative pentose phosphate pathways and relationship with glycolysis. Principal cellular functions of the pathways are indicated, and roles of NADPH included. G6PDH, glucose 6-phosphate dehydrogenase; PGDH, 6- 3 phosphogluconate dehydrogenase; RPE, ribulose-5-phosphate 3-epimerase; RPI, ribose 5-phosphate isomerase; TKL, transketolase. CH 4. HMP Hexose Monophosphate Pathway CH 4. HMP 4 Hexose Monophosphate Pathway It consists of two irreversible oxidative reactions, followed by a series of reversible sugar-phosphate interconversions No ATP is directly consumed or produced in the cycle. Carbon 1 of glucose 6-phosphate is released as CO2, and two NADPH are produced for each glucose 6-phosphate entering the oxidative part of the pathway. 5 CH 4. HMP The rate and direction of the reactions at any given time are determined by the supply of and demand for intermediates in the cycle. The HMP occurs in the cytosol of the cell. The pathway provides a major portion of the cell's NADPH, which functions as a biochemical reductant. The HMP also produces ribose-phosphate, required for biosynthesis of nucleotides, 6 CH 4. HMP for each molecule of glucose 6-phosphate oxidized. 1. Dehydrogenation of glucose 6-phosphate 2. Hydrolysis of 6- phosphogluconolactone and formation of ribulose 5- phosphate. The oxidative portion of the HMP leads to the formation of -two molecules of NADPH -CO2, and -ribulose 5-phosphate, 7 CH 4.