Austropuccinia Psidii on the Move: Survey Based Insights to Its Geographical Distribution, Host Species, Impacts and Management in Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two New Taxa of Verticordia (Myrtaceae: Chamelaucieae) from South-Western Australia
A.S.Nuytsia George 20: 309–318 & M.D. (2010)Barrett,, Two new taxa of Verticordia 309 Two new taxa of Verticordia (Myrtaceae: Chamelaucieae) from south-western Australia Alex S. George1 and Matthew D. Barrett2,3 1 ‘Four Gables’, 18 Barclay Road, Kardinya, Western Australia 6163 Email: [email protected] 2 Botanic Gardens and Parks Authority, Kings Park and Botanic Garden, Fraser Ave, West Perth, Western Australia 6005 3 School of Plant Biology, University of Western Australia, Crawley, Western Australia 6009 Email: [email protected] Abstract George, A.S. and Barrett, M.D. Two new taxa of Verticordia (Myrtaceae: Chamelaucieae) from south- western Australia. Nuytsia 20: 309–318 (2010). Verticordia mitchelliana subsp. implexior A.S.George & M.D.Barrett and Verticordia setacea A.S.George are described and discussed. Verticordia setacea belongs with V. gracilis A.S.George in section Platandra, previously a monotypic section. Introduction The genus Verticordia DC. (Myrtaceae: tribe Chamelaucieae) is a charismatic group of shrubs found mainly in south-western Australia, with several species in adjacent arid regions and three in tropical Australia (George 1991; George & Pieroni 2002). Verticordia is currently defined solely on the possession of divided calyx lobes, but the limits between Verticordia and the related genera Homoranthus A.Cunn. ex Schauer, Chamelaucium Desf. and Darwinia Rudge are difficult to define conclusively, and other characteristics such as anther morphology suggest conflicting relationships (Bentham 1867; Craven & Jones 1991; George 1991). A recent analysis using a single chloroplast gene, with limited sampling of Verticordia taxa (Ma et al. 2002), suggests that Verticordia may be polyphyletic. -

Wildflowers to Grow in Your Garden Here Is the Key to the List Large
Wildflowers to grow in your garden Here is the key to the list Trees Ground covers Shrubs Eucalypts Banksias Myrtle family Banksias Others Baeckea Other Beaufortia Calothamnus Chamelaucium Hypocalymna Kunzea Melaleuca and Callistemon Scholtzia Thryptomene Verticordia Large trees. Think very carefully before you plant them! Large trees, such as lemon scented gums or spotted gums may look great in parks - at least local councils seem to think so (we would rather see local plants). But you may regret planting them in a modern small garden. That doesn't mean there is no room for trees. There are hundreds of attractive small trees that grow very well in native gardens. Here are just a few. Small trees Eucalypts with showy flowers. Eucalytpus caesia Comes in two sub species with the one known as "silver princess" being readily available in Perth. Lovely multi- stemmed weeping tree with pendulous pink flowers and silver-bell fruits. E. torquata Small upright tree with attractive pink flowers. Very drought resistant. E. ficifolia Often called the WA Flowering gum. Ranges in size from small to quite large and in flower colour from deep red to = Corymbia ficifolia orange to pale pink. In WA subject to a serious disease - called canker. Many trees succumb when about 10 or so years old, either dying or becoming very unhealthy. E. preissiana Bell fruited mallee. Small tree (or shrub) with bright yellow flowers. E. erythrocorys Illyarrie, red cap gum or helmet nut gum. Large golden flowers in February preceded by a bright red bud cap. Tree tends to be bit floppy and to need pruning. -

Salinity Tolerance of Muntries (Kunzea Pomifera F. Muell.)
HORTSCIENCE 53(11):1562–1569. 2018. https://doi.org/10.21273/HORTSCI13280-18 When crops are subjected to soil salinity levels exceeding their tolerance levels, plant Kunzea growth declines and crop yields decrease. For Salinity Tolerance of Muntries ( example, strawberry (sensitive) exhibits a re- pomifera duced number of leaves and leaf area at F. Muell.), a Native Food Crop 30 mM NaCl and a 20% reduction in fruit yield (Garriga et al., 2015). A significant in Australia decrease in growth of date palm (tolerant) –1 is observed at 7.3 dS·m , and fruit yield is Chi M. Do, Kate L. Delaporte, Vinay Pagay, and Carolyn J. Schultz1 reduced by 25% (Department of Agricul- School of Agriculture, Food and Wine, Waite Research Institute, The ture and Food, 2016). Olive is an example of University of Adelaide, PMB1, Glen Osmond, SA, 5064, Australia a moderately tolerant fruit crop that shows relatively minor impacts at high salinity (7.5 Additional index words. alternative fruits, homeostasis, potassium, salinity stress, sodium dS·m–1), with 20% to 30% reduction in oil chloride, sustainable agriculture and fresh-fruit yield compared with nonsalt- stressed plants (Al-Rawi and Al-Mohemdy, Abstract . Identifying productive food crops that tolerate moderate soil salinity is critical 2001). In citrus (lime and lemon, both sensi- Kunzea pomifera for global food security. We evaluate the salinity tolerance of tive crops), moderate salinity (50 mM NaCl) (muntries), a traditional Indigenous food plant that grows naturally in coastal regions reduces leaf number, area, and thickness of southern Australia and thrives on relatively low rainfall. -

TML Propagation Protocols
PROPAGATION PROTOCOLS This document is intended as a guide for Tamborine Mountain Landcare members who wish to assist our regeneration projects by growing some of the plants needed. It is a work in progress so if you have anything to add to the protocols – for example a different but successful way of propagating and growing a particular plant – then please give it to Julie Lake so she can add it to the document. The idea is that our shared knowledge and experience can become a valuable part of TML's intellectual property as well as a useful source of knowledge for members. As there are many hundreds of plants native to Tamborine Mountain, the protocols list will take a long time to complete, with growing information for each plant added alphabetically as time permits. While the list is being compiled by those members with competence in this field, any TML member with a query about propagating a particular plant can post it on the website for other me mb e r s to answer. To date, only protocols for trees and shrubs have been compiled. Vines and ferns will be added later. Fruiting times given are usual for the species but many rainforest plants flower and fruit opportunistically, according to weather and other conditions unknown to us, thus fruit can be produced at any time of year. Finally, if anyone would like a copy of the protocols, contact Julie on [email protected] and she’ll send you one. ………………….. Growing from seed This is the best method for most plants destined for regeneration projects for it is usually fast, easy and ensures genetic diversity in the regenerated landscape. -

PHYTOCHEMICAL ANALYSIS and ANTIBACTERIAL ACTIVITY of EUCALYPTUS SP LEAF EXTRACT AGAINST CLINICAL PATHOGENS S.Sasikala and J
International Standard Serial Number (ISSN): 2249-6807 International Journal of Institutional Pharmacy and Life Sciences 4(6): November-December 2014 INTERNATIONAL JOURNAL OF INSTITUTIONAL PHARMACY AND LIFE SCIENCES Life Sciences Research Article……!!! Received: 27-10-2014; Revised: 31-10-2014; Accepted: 01-11-2014 PHYTOCHEMICAL ANALYSIS AND ANTIBACTERIAL ACTIVITY OF EUCALYPTUS SP LEAF EXTRACT AGAINST CLINICAL PATHOGENS S.Sasikala and J. Kalaimathi* Department of Biochemistry, Sri Akilandeswari womens College Wandiwash, TN, India Keywords: ABSTRACT Eucalyptus globulus, Medicinal plants are considerably useful and economically Medicinal palnt, essential. They contain active constituents that are used in the Antimicrobial activity treatment of many human diseases. Infectious diseases are world’s most important reason of untimely death, killing For Correspondence: 50,000 people each day. Resistance to antimicrobial agents is J. Kalaimathi rising in a wide diversity of pathogens and numerous drug Department of Biochemistry, resistances are becoming common in diverse organisms. The Sri Akilandeswari womens plant extracts have been developed and proposed for use as College Wandiwash, TN, India antimicrobial substances. Many of the plant materials used in E-mail: traditional medicine are readily available in rural areas at relatively cheaper than modern medicine. The present study [email protected] was aimed to evaluate the antibacterial potential of methanol extract of Eucalyptus globulus against bacterial pathogens and phytochemical analysis was done. 47 Full Text Available On www.ijipls.com International Standard Serial Number (ISSN): 2249-6807 INTRODUCTION In the production of drugs, the role of plants is very important. There is a lot of drugs are produced from the plants and its various parts (Fabricant and Farnsworth 2001, Farnsworth et al., 19858) . -

An Infrageneric Classification of Syzygium (Myrtaceae)
Blumea 55, 2010: 94–99 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651910X499303 An infrageneric classification of Syzygium (Myrtaceae) L.A. Craven1, E. Biffin 1,2 Key words Abstract An infrageneric classification of Syzygium based upon evolutionary relationships as inferred from analyses of nuclear and plastid DNA sequence data, and supported by morphological evidence, is presented. Six subgenera Acmena and seven sections are recognised. An identification key is provided and names proposed for two species newly Acmenosperma transferred to Syzygium. classification molecular systematics Published on 16 April 2010 Myrtaceae Piliocalyx Syzygium INTRODUCTION foreseeable future. Yet there are many rewarding and worthy floristic and other scientific projects that await attention and are Syzygium Gaertn. is a large genus of Myrtaceae, occurring from feasible in the shorter time frame that is a feature of the current Africa eastwards to the Hawaiian Islands and from India and research philosophies of short-sighted institutions. southern China southwards to southeastern Australia and New One impediment to undertaking studies of natural groups of Zealand. In terms of species richness, the genus is centred in species of Syzygium, as opposed to floristic studies per se, Malesia but in terms of its basic evolutionary diversity it appears has been the lack of a framework or context within which a set to be centred in the Melanesian-Australian region. Its taxonomic of species can be the focus of specialised research. Below is history has been detailed in Schmid (1972), Craven (2001) and proposed an infrageneric classification based upon phylogenies Parnell et al. (2007) and will not be further elaborated here. -

Muelleria : an Australian Journal of Botany
Muelleria Volume 5 Number 1 March, 1982 NATIONAL HERBARIUM OF VICTORIA DEPARTMENT OF CROWN LANDS AND SURVEY Muelleria Volume 5, Number 1 March, 1982 CONTENTS Page A revision of the genus Templelonia R.Br. (Papilionaceae) — J. H. Ross 1 The nomenclature of some Australian lichens described as Lecanora and Placodium by Miiller-Argoviensis — R. W. Rogers 31 New Australian species of Nymphoides Seguier (Menyanthaceae) — Helen 1. Aston 35 Vegetation of East Gippsland — S. J. Forbes, N. G. Walsh and P. K. Gullan 53 A new Australian lichen: Cladonia sulcata — A. W. Archer 115 Editor: Helen 1. Aston Published by the National Herbarium of Victoria (MEL). Royal Botanic Gardens, South Yarra, Victoria 3141, Australia. D. M. Churchill, Director and Government Botanist. 43346/81 The date of publication of Volume 4, number 4, was 20 May 1981. A REVISION OF THE GENUS TEMPLETONIA R.Br. (PAPILIONACEAE) by J. H. Ross* ABSTRACT The endemic Australian genus Templetonia is revised. Eleven species are recognized and the uncertainty concerning the application of the name T. sulcata (Meissn.) Benth. is discussed. This discussion includes the selection ol a lectotype for Bossiaea rossii F. Muell., a possible synonym. Descriptions, a key to the identification of species, illustrations, and distribution maps are provided, together with notes on ecology and relationships. Two previous papers describing T. incana (.Muelleria 4: 247-249 (1980)) and T. negketa (loc. cit. 390-393 (1981)) should be used in conjunction with the present revision. INTRODUCTION Templetonia, a small genus of 1 1 species described by R. Brown in Ait. f Hort. , Kew. ed. 2, 4: 269 (1812), was named in honour of the Irish botanist John Templeton (1776-1825) ot Orange Grove, Belfast. -

Lauraceae Along an Altitudinal Gradient in Southern Brazil
Floresta e Ambiente 2019; 26(4): e20170637 https://doi.org/10.1590/2179-8087.063717 ISSN 2179-8087 (online) Original Article Forest Management Lauraceae Along an Altitudinal Gradient in Southern Brazil Marcelo Leandro Brotto1 , Eduardo Damasceno Lozano2 , Felipe Eduardo Cordeiro Marinero2 , Alexandre Uhlmann3 , Christopher Thomas Blum2 , Carlos Vellozo Roderjan2 1Prefeitura Municipal de Curitiba, Curitiba, PR, Brasil 2Universidade Federal do Paraná (UFPR), Curitiba, PR, Brasil 3Embrapa Pesca e Aquicultura, Palmas, TO, Brasil ABSTRACT We performed a phytosociological study on an altitudinal gradient in Lauráceas State Park (Parque Estadual das Lauráceas/PR), aiming to describe the Montane Atlantic Rain Forest, to verify the importance of Lauraceae, and to evaluate the communities’ successional stage. We distributed survey units (2,000 m² quadrats) along an altitudinal gradient and surveyed all individuals with DBH ≥ 10 cm, which composed the arboreal component. In smaller quadrats (250 m²), we surveyed regeneration individuals. The community at 800 and 900 m a.s.l. shows typical characteristics of Montane forest in an advanced successional stage, and the abundance of Ocotea catharinensis is its main indicator. At 1,000 and 1,100 m a.s.l., the forest is characterized as Montane with short stature in an advanced successional stage, with the occurrence of typical upper montane species such as O. porosa and O. vaccinioides. Keywords: Atlantic forest, Lauráceas State Park, phytosociology. Creative Commons License. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. 2/12 Brotto ML, Lozano ED, Marinero FEC, Uhlmann A, Blum CT, Roderjan CV Floresta e Ambiente 2019; 26(4): e20170637 1. -
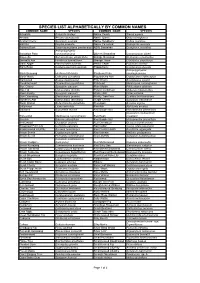
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Pre-Infection Stages of Austropuccinia Psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels
Article Pre-Infection Stages of Austropuccinia psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels Renata Ruiz Silva-Souza 1, André Costa da Silva 2, Roberto Antônio Rodella 3, José Eduardo Serrão 4, José Cola Zanuncio 5 and Edson Luiz Furtado 1,* 1 Departamento de Produção Vegetal, Faculdade de Ciências Agronômicas–UNESP, Botucatu 18610-307, São Paulo, Brasil; [email protected] 2 Departamento de Fitopatologia, Universidade Federal de Viçosa, Viçosa 36570-900, Minas Gerais, Brasil; [email protected] 3 Departamento de Botânica, Instituto de Biociências de Botucatu–UNESP, Botucatu 18618-000, São Paulo, Brasil; [email protected] 4 Departamento de Biologia Geral, Universidade Federal de Viçosa, Viçosa 36570-900, Minas Gerais, Brasil; [email protected] 5 Departamento de Entomologia/BIOAGRO, Universidade Federal de Viçosa, Viçosa 36570-900, Minas Gerais, Brasil; [email protected] * Correspondence: [email protected]; Tel.: +55-31-3899-2924 Received: 25 July 2017; Accepted: 20 September 2017; Published: 25 September 2017 Abstract: Rust is a major Eucalyptus spp. disease, which is especially damaging for early-stage plants. The aim of this study was to verify the pre-infection process of Austropuccinia psidii (A. psidii) in the leaves of three phenological stages of Eucalyptus clones with different resistance levels. Plants from the hybrids of Eucalyptus urophylla × Eucalyptus grandis (E. grandis) with variable levels of resistance to this disease were used. The pathogen was inoculated in vitro on abaxial leaf discs of first, third, and fifth leaf stages and maintained under conditions suitable for disease development. Subsequently, samples from these discs were collected 24 and 120 h after inoculation and processed using scanning electron microscopy analysis. -

Myrtle Rust Resource for Gardeners
Resource for Gardeners Know myrtle rust – and don’t touch it! Myrtle rust forms pustules of tiny yellow spores. These spores can stick to clothes, shoes and fingers and spread to other plants – so please don’t touch it! In plants where the disease has progressed, pustules become white, then grey and infected plant material dies and turns brown/grey. For more information on myrtle rust, please visit myrtlerust.org.nz or the Beyond Myrtle Rust website. If you suspect a plant is infected with myrtle rust, please take a photo and upload it to iNaturalistNZ. Ramarama Brush cherry Rōhutu Lophomyrtus bullata Syzygium australe Lophomyrtus obcordata Know your myrtles Native myrtles include pōhutukawa, mānuka, kānuka, ramarama and rātā. Non- native myrtles include feijoa, eucalypts, bottlebrush, guavas, willow myrtle, lilly pilly and monkey apple. If you are unsure whether your plant is from the myrtle family, you can use the NZ Myrtaceae key to help identify it or upload a picture onto iNaturalistNZ for confirmation. You can also ask for more information on myrtles and myrtle rust at your local nursery or botanical garden. Garden plants that are particularly susceptible to myrtle rust are Syzygium species (monkey apple and lilly pillys) and Lophomyrtus. Here is a list of myrtle species that have been found with myrtle rust in New Zealand. Prune myrtles in winter Myrtle rust spores only infect new growth, including leaves, stems and flower buds. Since pruning stimulates new growth, it is best to time pruning for when the disease is least infectious – in the winter (June, July and August), not in summer. -

Austropuccinia Psidii
This diagnostic protocol was adopted by the Standards Committee on behalf of the Commission on Phytosanitary Measures in August 2018. The annex is a prescriptive part of ISPM 27. ISPM 27 Diagnostic protocols for regulated pests DP 26: Austropuccinia psidii Adopted 2018; published 2018 CONTENTS 1. Pest Information ...............................................................................................................................2 2. Taxonomic Information ....................................................................................................................2 3. Detection ...........................................................................................................................................4 3.1 Signs and symptoms of infection ......................................................................................4 3.2 Sampling and sample preparation .....................................................................................5 3.3 Morphological detection ...................................................................................................5 3.4 Molecular detection ...........................................................................................................6 3.4.1 Preparation of plant material .............................................................................................6 3.4.2 Nucleic acid extraction ......................................................................................................6 3.4.3 Conventional PCR and sequencing ...................................................................................7