9088 Medical Single
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Provider Section
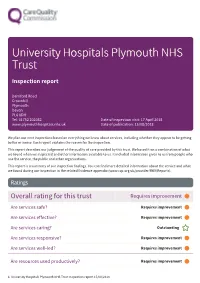
University Hospitals Plymouth NHS Trust Inspection report Derriford Road Crownhill Plymouth Devon PL6 8DH Tel: 01752 202082 Date of inspection visit: 17 April 2018 www.plymouthhospitals.nhs.uk Date of publication: 15/08/2018 We plan our next inspections based on everything we know about services, including whether they appear to be getting better or worse. Each report explains the reason for the inspection. This report describes our judgement of the quality of care provided by this trust. We based it on a combination of what we found when we inspected and other information available to us. It included information given to us from people who use the service, the public and other organisations. This report is a summary of our inspection findings. You can find more detailed information about the service and what we found during our inspection in the related Evidence appendix (www.cqc.org.uk/provider/RK9/Reports). Ratings Overall rating for this trust Requires improvement ––– Are services safe? Requires improvement ––– Are services effective? Requires improvement ––– Are services caring? Outstanding Are services responsive? Requires improvement ––– Are services well-led? Requires improvement ––– Are resources used productively? Requires improvement ––– 1 University Hospitals Plymouth NHS Trust Inspection report 15/08/2018 Summary of findings Combined quality and resource rating Requires improvement ––– We rated well-led (leadership) from our inspection of trust management, taking into account what we found about leadership in individual services. We rated other key questions by combining the service ratings and using our professional judgement. Background to the trust Background information University Hospitals Plymouth NHS Trust (formerly, until 1 April 2018, Plymouth Hospitals NHS Trust) is the largest hospital trust in the south west peninsula. -

Derriford Research Facility 2 3
DERRIFORD RESEARCH FACILITY 2 3 UNIVERSITY OF PLYMOUTH FACULTY OF MEDICINE AND DENTISTRY With three core aims at the heart of everything we do, • Our research output in clinical medicine is ranked top in the UK, with 80% exceptional clinical learning, strong social engagement and of the research classed as ‘internationally excellent’ or ‘world leading* world class research, the Faculty has earned a strong and enviable track record for delivering high-calibre training for • The University of Plymouth Brain Tumour Research Centre of Excellence, a wide range of health professionals. Training more than led by Professor Oliver Hanemann, is one of only four in the UK funded 1,800 students and professionals each year, drives forward by Brain Tumour Research. It is playing a key role in researching new the vision for Plymouth as the ‘First Choice for Health’ in the South West. treatments for brain tumours, the biggest cancer killer of children and adults under the age of 40, and tumours of the nervous system Through our graduates and research we celebrate our vision. In addition, we see this recognised through various • The significance of our cross-discipline approach in Parkinson’s disease accolades and privileges. We enjoy ‘top three’ national is recognised through the appointment of Dr Camille Carroll as National status for preparedness for both newly qualified doctors and Institute for Health Research (NIHR) Clinical Research Network (CRN) their clinical supervisors (General Medical Council national Training Survey), and for our dental school (The Guardian National Specialty Lead for Neurodegeneration. There are 127,000 University Guide 2018), whilst our dynamic and rapidly people with Parkinson’s in the UK with one more person diagnosed expanding research operation and reputation continues to every hour shine a spotlight on just how much this comparatively young Faculty is achieving. -

Travelling to Derriford Hospital by Bus and Train from South and West Devon and East Cornwall
120990 Travelling to Derriford 11/6/07 10:04 am Page 1 Travelling to Derriford Hospital by bus and train from South and West Devon and East Cornwall from 4th June 2007 120990 Travelling to Derriford 11/6/07 10:04 am Page 2 Welcome... Travelling to Derriford ...to this easy to use guide to Hospital from Central travelling to Derriford Hospital by Plymouth bus and train from South and West Devon and East Cornwall. It By Bus includes summary details of direct bus and rail services to Plymouth If your bus into Plymouth terminates at Bretonside Bus Station, First services 83, 84 and 86 and details of connecting buses to run every 20 minutes to Derriford from stand 4. Derriford. There are also several routes from Royal Parade in the City Centre to Derriford Hospital, with First service 7 and Plymouth Citybus 42/A/B being the How to use this guide most direct. The 7 runs every 20 minutes and the 42/A/B every 30 minutes. Both leave from stop The coloured map on pages 4-5 shows services A18, near Debenhams. to Plymouth from areas around the city. The frequency guide gives a summary of If you are travelling from East Cornwall you may frequencies for these services. be able to change buses at St Budeaux and catch Plymouth Citybus 29 to Derriford. The 29 runs Most bus and train services operate to every 30 minutes. Plymouth city centre and will require a connecting bus journey to Derriford Hospital. Details of buses from the city centre are shown By Train on page 3. -

University Hospitals Plymouth NHS Trust
CASE STUDY University Hospitals Plymouth NHS Trust The Customer The Challenge University Hospitals Plymouth NHS Trust (UHPNT) is a large For an organisation that provides 24hr emergency care to acute district general hospital, based in south west Devon. It is over 2,000,000 people in the south west peninsula, reliable the largest hospital in the south west peninsula, providing the network connectivity is a critical factor. Hospital staff rely on region with comprehensive secondary and tertiary healthcare the network to access patient records and information as well services. Beyond its provision of secondary services to some as clinical applications that run over the network. Resilient 450,000 people from Plymouth and the surrounding area, connectivity ensures that staff are informed and well equipped UHPNT is a major trauma centre and provides tertiary and to treat patients in potentially life-threatening situations. specialist services to a population of over 2,000,000 people. Up until April 2017, UHPNT was wholly reliant on the UHPNT works in conjunction with Devon and Cornwall’s four N3 network, the national broadband network for all NHS other major hospitals and additional healthcare services in the organisations. This included supporting many applications region. Operating within this network of hospitals has enabled that were instrumental to providing patient care. The N3, the Trust to offer a greater range of specialist services such as introduced in 2006, became outdated over time and the initial kidney transplants, cardiothoracic surgery, neonatal intensive provision of 100MB bandwidth failed to meet its needs. What’s care and high-risk obstetrics. more, UHPNT’s N3 connection could not guarantee the low latency connection required to ensure the seamless and quick UHPNT’s main sites are: transfer of patient information across the network. -

South West Peninsula
Unit Brief v1 ICM Unit Brief Part 1 Hospital Details 1.1 Hospital name Derriford Hospital 1.2 Full address (you must include postcode) 1.3 Hospital Telephone number University Hospitals Plymouth NHS Trust 01752 202082 Derriford Road Crownhill Plymouth Devon PL6 8DH Part 2 ICU Department contact details 2.1 Direct telephone number to Department 01752 431404 (unit secretary) 2.2 Faculty Tutor name 2.3 Faculty Tutor Email address Dr Paul Margetts [email protected] Part 3 Unit Structure 3.1 Number of Beds 3.2 Number of admissions 28 1600-1700 3.3 Percentage of elective vs emergency admissions 29% High risk elective surgery 19% Emergency surgery 52% Non-surgical Page 1 (of 4) Unit Brief v1 3.4 Overview of case mix within the unit Plymouth Hospitals NHS Trust is one of the largest providers of acute care in the country. It is the tertiary referral centre and Major Trauma Centre for Devon, Cornwall and part of Somerset serving a population of almost 2 million. All services are provided on a single site at Derriford Hospital. These services include 3.neurosurgery,4 Details of trainingmaxillofacial opportunities surgery, upper on the and unit lower GI surgery, plastic surgery, renal transplantation, hepatobiliary surgery and cardiothoracic surgery. Over 1600 patients are admitted per annum in a purpose built 28 bed combined general and neuro intensive care unit. There is a separate 4 bed paediatric HDU adjacent to the paediatric wards and a 16 bed cardiac intensive care unit for patients following cardiac surgery. The general unit admits a small number of children a year, but most are retrieved to the PICU in Bristol following stabilisation in Plymouth. -

Acuity Audit of Hospital Bed Occupancy in Devon, 2018
Acuity Audit of Hospital Bed Occupancy in Devon June 2018 Table of Contents Table of Figures Executive Summary 1. Introduction .................................................................................................... 2 2. Background ................................................................................................... 2 3. Method .......................................................................................................... 5 4. Results .......................................................................................................... 6 5. Discussion ................................................................................................ ...10 6. Summary of Findings ................................................................................... 19 7. Conclusions ................................................................................................. 21 Table of Figures Figure 1: Version Control of audit tool used……………………………………………....………4 Figure 2: Overall patient numbers and patients ‘fit to leave’ by locality and hospital type….7 Figure 3: Patient age by hospital type and ‘fit to leave’ status .... ………………………………7 Figure 4: Analysis of ‘fit to leave’ patients, who could be managed at home, with further needs ……………………………………………………………………………………………..…..8 Figure 5: Number of days medically fit – number of patients………………………………..….8 Figure 6: Analysis of the reason in bed patients, conducted on patients classed as ‘fit to leave’. …………………………………………………………………………………………..…….8 Figure 7: Analysis -

Plymouth Network
Plymouth Network 1 to Yelverton & Tavistock dayrider & megarider zone includes Tesco Roborough on route 1 100101 Plymouth Inner George Junction park&ride Glenholt 14 Marjon University Burraton Latchbrook Derriford Higher Hospital 2A St Budeaux HM Land Registry Saltash 2 2A Saltash Passage Crownhill St Budeaux Brake Saltash Victoria Farm Burraton Rd Coombe Hartley Vale 2 Lidl 31 St Stephens Longbridge buses use these stops in both directions Barne Morrisons 200 Barton Pennycross Woodford Coypool Marsh Mills park&ride Retail Park Colebrook Hartley Keyham HMS Drake Plympton Keyham Milehouse Peverell park&ride Efford Merafield Plymouth Argyle FC Central Park 19 Plymouth College Mutley Laira Dockyard Independent Lipson Community Ford College Milehouse Park Devonport Aldi to Buckfastleigh Mount Gould from Bristol X Plymouth Hospital towards Bristol & Exeter 38 Devonport Mount to Totnes, Paignton & Torquay Stoke Gould Devonport Park Plymouth Inner to Bristol Torpoint dayrider & megarider zone includes 36 Ivybridge on X38 & Gold Plymouth CityCentre Sugar Mill Business Park Billacombe Prince Sherford 2A National Rock Morrisons Pomphlett The Hoe Marine Aquarium from Bristol 2A towards Bristol Barbican Cattedown Lidl Mount Batten Centre Plymstock The Broadway Dolphin Cremyll Co-op Mount Batten 2 Turnchapel Court Rd Elburton 3 to Kingsbridge & Hooe Dartmouth where to catch your bus in Plymouth City Centre route towards city centre stops Plymouth Tavistock via Derriford Hospital S4 M1 W3 W6 C U S2 1 A21 1 2 SALTASH bus stops Saltash 2 2A B5 A6 W4 -

University Hospitals Plymouth NHS Trust
University Hospitals Plymouth NHS Trust Use of Resources assessment report Derriford Road, Crownhill, Plymouth, PL6 8DH Tel: 01752 202082 www.plymouthhospitals.nhs.uk Date of publication: 15 August 2018 This report describes our judgement of the Use of Resources and our combined rating for quality and resources for the trust. Ratings Overall quality rating for this trust Requires improvement Are services safe? Requires improvement Are services effective? Requires improvement Are services caring? Outstanding Are services responsive? Requires improvement Are services well-led? Requires improvement Our overall quality rating combines our five trust-level quality ratings of safe, effective, caring, responsive and well-led. These ratings are based on what we found when we inspected, and other information available to us. You can find information about these ratings in our inspection report for this trust and in the related evidence appendix. (www.cqc.org.uk/provider/RK9/Reports) Are resources used productively? Requires improvement Combined rating for quality and use of Requires improvement resources We award the Use of Resources rating based on an assessment carried out by NHS Improvement. Our combined rating for Quality and Use of Resources summarises the performance of the trust taking into account the quality of services as well as the trust’s productivity and sustainability. This rating combines our five trust-level quality ratings of safe, effective, caring, responsive and well-led with the Use of Resources rating. Page 1 of 15 Use of Resources assessment and rating NHS Improvement are currently planning to assess all non-specialist acute NHS trusts and foundation trusts for their Use of Resources assessments. -
Kathy Webb Winner of the Dr Kate Granger Care and Compassion Award 2018
AUTUMN 2018 East Cornwall & West Devon Network KATHY WEBB WINNER OF THE DR KATE GRANGER CARE AND COMPASSION AWARD 2018 Wouldn’t be Kathy has got where I am me through now without some very tough her!! times You are simply IBD nurse of the best Kathy x the decade for us!! Thank you for all you have done and Truly an amazing continue to do as it woman! Thank you for means more than you everything you do for would ever know x me and everyone else! Congratulations Kathy you Couldn't be more really deserve it. Not only deserving. Extremely are you amazing at your compassionate and a job but you are so lovely true professional. with it x We were delighted to hear that Kathy had won the Dr Kate Granger Care and Compassion Award for 2018 … and so were you! There were so many congratulatory comments on our posts that we couldn’t possibly reproduce them all here so this is just a sample. It’s an overwhelming expression of love and gratitude for an IBD nurse who goes above and beyond. THANK YOU KATHY Crohn’s and Colitis UK East Cornwall & West Devon Network Newsletter AUTUMN 2018 Page 2 COMEDY EVENING RAISES ALMOST £9,000! Meet Ian Pearson and Charlotte Bethany (left) who have raised an amazing £8,886 for Crohn’s & Colitis UK! Ian, who is an inveterate fundraiser, approached Charlotte after he saw her post on social media about her own fundraising at Plymouth Walk It. He wanted to create something that would raise money while having fun so came up with the idea for a Comedy Evening. -

Candidate Information Pack
Candidate Information Pack Job Title: Consultant Cellular Pathologist Reference Number: 216-9664-PATH Closing Date: as specified on NHS Jobs Dear candidate, Thank you for your interest in this post and for taking the time to read through our information pack and advert. If you have any remaining questions, please do get in touch with us. This is an exciting opportunity to work in the largest hospital trust in the South West Peninsula. We are a teaching hospital in partnership with the Peninsula Medical School and are rapidly developing as a centre for innovation and research. We employ 6400 staff, have more than 900 beds, and over 48,000 people pass through the main entrance of our hospital in a week. In addition, we have an integrated Ministry of Defence Hospital Unit, which has a staff of approximately 250 military personnel. Plymouth, Britain’s Ocean City, occupies a stunning location. It’s a perfect city for ambitious people looking to build a career, and enjoy a rich and rewarding life. Please follow the link below to read through Plymouth’s Book of Wonder and find out more: http://www.visitplymouth.co.uk/ Everything we do is guided by our core values: Put Patients First Take Ownership Respect Others Be Positive Listening, Learning, Improving We very much look forward to receiving your application and will be in touch via NHS Jobs if you have been shortlisted for interview. If you do not hear from us within 4 weeks of the closing date then please note you have not been successful on this occasion. -

Minutes Wednesday 4Th July 2018: 2:00 Pm – 4.30 Pm the Watermark, Erme Court, Leonards Road, Ivybridge PL21 0SZ
Meeting of the South and West Devon Formulary Interface Group Minutes Wednesday 4th July 2018: 2:00 pm – 4.30 pm The Watermark, Erme Court, Leonards Road, Ivybridge PL21 0SZ Present: Andrew Gunatilleke (Chair) Consultant Torbay & South Devon NHS FT Trudy Bown Chief Pharmacy Procurement University Hospitals NHS Plymouth & IT Manager Emma Gitsham Joint Formularies Pharmacist NEW Devon CCG Lily Hammarlund-Sim Pharmaceutical Advisor Kernow CCG Matt Howard Clinical Evidence Manager NEW Devon CCG Nicola Joyce Pharmacist Livewell Southwest Sarah Marner Interface MO Pharmacist NEW Devon CCG Phil Melluish GP South Devon & Torbay CCG Bill Nolan GP South Devon & Torbay CCG Hilary Pearce Clinical Effectiveness Pharmacist NEW Devon CCG Iain Roberts Lead MO Pharmacist South Devon & Torbay CCG Christopher Sullivan Pharmacist Devon Partnership NHS Trust Darren Wright Joint Formularies Technician NEW Devon CCG Guests: Charlotte Carvell Senior Clinical Pharmacist University Hospitals Plymouth NHS (Rheumatology, TOT, Thrombosis Trust (UHPNT) & Anticoagulation) Edward Davies Consultant Cardiologist University Hospitals Plymouth NHS Trust Rosie Fok Consultant Microbiologist & University Hospitals Plymouth NHS Antimicrobial Stewardship Lead Trust (UHPNT) Theresa Mitchell Tissue Viability Clinical Nurse Livewell Southwest Tony Perkins Lead Respiratory Pharmacist Livewell Southwest Sarah Jane Rowlands Practice Pharmacist South Devon and Torbay CCG Larissa Sullivan Lead Pharmacist – Long Term Torbay & South Devon NHS FT Conditions In attendance: Fiona Dyroff Clinical Effectiveness NEW Devon CCG Governance Support Officer 1 1. Welcome and announcements Welcome and announcements Attendees were welcomed to the meeting. Apologies Andy Craig GP NEW Devon CCG Paul Foster Clinical Director - Pharmacy & Prescribing Torbay & South Devon NHS Foundation Trust Josh Hamilton GP Kernow CCG Peter Rowe Consultant Nephrologist University Hospitals Plymouth NHS Trust Mark Stone Community Pharmacist Declaration of Interests Declarations of Interest were collected and reported. -

Contact & Location Information
CONTACT & LOCATION INFORMATION CONTACT & LOCATION INFORMATION T: 01752 437837 E: [email protected] W: www.peninsulaheartclinic.co.uk World‐class cardiology services in partnership with University Hospitals Plymouth How to Find us Peninsula Heart Clinic University Hospitals Plymouth NHS Trust Derriford Hospital Derriford Road Car Parking Plymouth We have 21 parking spaces in a pay and display car park directly next to our heart clinic, this includes 4 disabled Devon PL6 8DH parking bays. We recommended patients or visitors park in United Kingdom this car park as this is the closest to the heart clinic. Parking is also available in a number of car parks across the Derriford hospital campus. If you park in the large multi-storey (Bircham) car park it is a short walk down the road past the How to find us maternity centre on the left to the heart clinic. Alternatively, By Car you may wish to follow signs to the main entrance where there will be signposting. If you are a private patient visiting From roundabout on A386 take exit signposted Derriford our outpatients suite or being admitted for a planned Hospital. As you enter the main entrance you will see a procedure, please contact our team.We also provide a patient ‘Peninsula Heart Clinic’ sign directing you to the right hand side drop-off area consisting of 4 car parking spaces directly of the main hospital. You will see the orthopaedic centre directly outside the clinic, but parking is limited in this area to 15 ahead with the multi-story car park on the right hand side.