Study of Lignin to Value Added Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Electrocatalysts for Low-Temperature Fuel Cells Francisco Javier Rodríguez-Varela Teko W
Advanced Electrocatalysts for Low-Temperature Fuel Cells Francisco Javier Rodríguez-Varela Teko W. Napporn Editors Advanced Electrocatalysts for Low-Temperature Fuel Cells Editors Francisco Javier Rodríguez-Varela Teko W. Napporn Cinvestav CNRS Ramos Arizpe, Coahuila, Mexico French National Center for Scientific Research Poitiers, France ISBN 978-3-319-99018-7 ISBN 978-3-319-99019-4 (eBook) https://doi.org/10.1007/978-3-319-99019-4 Library of Congress Control Number: 2018957465 © Springer International Publishing AG, part of Springer Nature 2018 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Technical and Scientific Half Year Progress Report
NMP-CT-2003-505567 BIOCELSOL Biotechnological Process for Manufacturing Cellulosic Products with Added Value Instrument: Specific Targeted Research Project (STREP) Thematic Priority: Nanotechnology and nanosciences, knowledge based multifunctional materials, new production processes and devices (NMP) FINAL REPORTS Part of D43, Public Publishable final activity report including publishable project results, and D29 Summary report of Interim progress reports (PU) Period covered: from 1.3.2004 to 28.2.2007 (project months 1–36) Start date of project: 1st March 2004 Duration: 36 months Project co-ordinator organisation name: Tamlink Ltd Project co-ordinator name: Sirkku Hoikkala Date of preparation: 12th April 2007 Revision: [ final version ] BIOCELSOL NMP2-CT-2003-505567 Publishable final activity report Introduction A novel biotechnology-based method for converting cellulose into fibres, films, casings, beads and sponges was studied and further developed during the Biocelsol project (1.3.2004 – 28.2.2007). In the method the dissolving grade pulp is modified to an alkali soluble form by treating it with enzymes. The treated pulp is thereafter dissolved and the cellulose solution regenerated into desired shapes. No hazardous and toxic carbon disulphide (CS2), which is used in the viscose method, is needed and thus no emissions to the air are liberated, contrary to the viscose process. The methodology used included the optimisation of 1) enzymatic action on cellulose, 2) dissolution technique of treated cellulose and 3) regeneration of alkaline cellulose solution. The research work was carried out in laboratory scale in five groups, which focused on the pulp treatments (WP1), dissolution of treated pulp (WP2), development of fibre spinning process (WP3), development of film and fibrous casings production processes (WP4) and development of beads and sponges production processes (WP5). -

I~ Boreskov Institute of Catalysis Siberian Branch of Russian Academy of Sciences
I~ BORESKOV INSTITUTE OF CATALYSIS SIBERIAN BRANCH OF RUSSIAN ACADEMY OF SCIENCES ANNUAL REVIEW of Activities in Fundamental Areas 1999 BORESKOV INSTITUTE OF CATALYSIS SIBERIAN BRANCH OF RUSSIAN ACADEMY OF SCIENCES Novosibirsk Published by Boreskov Institute of Catalysis Pr. Akademika Lavrentieva, 5 Novosibirsk, 630090 Russia Phone: (007) 383 2 34 32 69 FAX: (007) 383 2 34 30 56 E-mail: [email protected] http: //www.catalysis.nsk.su © Boreskov Institute of Catalysis, 2000 2 Dear Colleagues, This is a real pleasure for me to present already the sixth issue of annual reviews of the Boreskov Institute of Catalysis in the main fields of its academic and R&D activities covering the year 1999. The Boreskov Institute of Catalysis is well known to experts in both academic and industrial catalysis not only in Russia and CIS, but also in many western and oriental countries. Since the sixties it has remained the largest chemical research institution in the Asian part of Russia having many deep and stable traditions and providing research in nearly all fields of catalysis. Its interests spread from the sophisticated purely academic research to the real applied problems, including commercial catalysts and catalytic technologies. During the last years according to numerous changes in Russia and its stepwise approach to the market economy, there were also some sufficient changes in the behavior and life of the Boreskov Institute of Catalysis. However, in spite of general instability in the country, the Institute could survive as a high quality specialized organization. Moreover, the intensity and quality of the research and engineering activity at the Institute became even higher. -

Challenges and Opportunities in Materials for Green Energy Production and Conversion
CONFERENCES VIRTUAL MEETING | 2021 15 - 17 June 2021 Challenges and opportunities in materials for green energy production and conversion LOCATION VIRTUAL MEETING CONVENORS Dr Edurne Serrano- Larrea FORMER LE STUDIUM RESEARCH FELLOW / ARD 2020 - LAVOISIER PROGRAMME Mineralogy and Petrology Department, UPV/ EHU - ES Dr Conchi Ania & Dr Encarnacion Raymundo-Piñero Extreme Conditions and Materials: High Temperature and Irradiation (CEMHTI) / CNRS - FR CONFERENCES VIRTUAL MEETING | 15-17 JUNE 2021 ABSTRACTS Challenges and opportunities in materials for green energy production and conversion CONVENORS Dr Edurne Serrano-Larrea FORMER LE STUDIUM RESEARCH FELLOW / LAVOISIER ARD 2020 PROGRAMME FROM: Mineralogy and Petrology Department, UPV/EHU - ES Dr Conchi Ania Extreme Conditions and Materials: High Temperature and Irradiation (CEMHTI) / CNRS - FR Dr Encarnacion Raymundo-Piñero Extreme Conditions and Materials: High Temperature and Irradiation (CEMHTI) / CNRS - FR ORGANIZING COMMITTEE Sophie Gabillet, General Secretary Dr Aurélien Montagu, Scientific Relations Manager Maurine Villiers, Communication & Events Manager LE STUDIUM Loire Valley Institute for Advanced Studies • Région Centre-Val de Loire • FR LE STUDIUM CONFERENCES Challenges and opportunities in materials for green energy production and conversion |15-17 June 2021 |3 EDITO Created in 1996 on the CNRS campus in Orleans La Source, LE STUDIUM has Development 2020 programmes initiated by the Centre-Val de Loire Regional evolved to become the multidisciplinary Loire Valley Institute for Advanced Council to support the smart specialisation strategy (S3) around 5 main axes: Studies (IAS), operating in the Centre-Val de Loire region of France. LE biopharmaceuticals, renewable energies, cosmetics, environmental metrology STUDIUM has its headquarters in the city centre of Orleans in a newly renovated and natural and cultural heritage. -

Pedro Arcelus Thesis
Heavy Oil Upgrading through Oxidative Cracking in Near-critical and Supercritical Water Pedro Manuel Arcelus Arrillaga Imperial College London Chemical Engineering Department Thesis submitted for the degree of Doctor of Philosophy (PhD) to Imperial College London Supervised by: Dr. Marcos Millan Agorio Prof. Klaus Hellgardt Declaration of Originality Declaration of Originality: The author declares that the contents of this work are the product of his original effort and that anything else has been appropriately referenced. Pedro Manuel Arcelus Arrillaga Copyright Declaration: The copyright of this thesis rests with the author and is made available under a Creative Commons Attribution Non-Commercial No Derivatives licence. Researchers are free to copy, distribute or transmit the thesis on the condition that they attribute it, that they do not use it for commercial purposes and that they do not alter, transform or build upon it. For any reuse or redistribution, researchers must make clear to others the licence terms of this work. Abstract Abstract The gradual decline in conventional oil production combined with an increasing world energy demand has made the production and upgrading of heavy and extra heavy oil feedstocks crucial for the future of the global energy market. Properties of heavy oil such as high viscosity and high specific gravity as well as the high percentage of asphaltenes, heteroatoms and metals cause severe problems during extraction and refining processes. As a result, traditional upgrading technologies are not suitable as a standalone method to process these feedstocks. This makes necessary the development of alternative or complementary technologies to process heavy oil feedstocks in a more efficient and environmentally friendly way. -

Pl. Inv. CISOPC Lm 2015-2016 Eng
Field: Chemical Engineering valid beginning with Academic Year 2015-2016 Specialization: Chemistry and Engineering of Organic Substances, Petrochemistry and Carbochemistry, Language of instruction: Hungarian Curriculum valid beginning with Academic Year 20152016 III. NUMBER OF WEEKLY HOURS BABEBOLYAI UNIVERSITY OF CLUJNAPOCA Year of Study 1st Semester 2nd Semester FACULTY OF CHEMISTRY AND CHEMICAL ENGINEERING 1st Year 24 28 Field of study: CHEMICAL ENGINEERING 2nd Year 25 28 Specialization: CHEMISTRY AND ENGINEERING OF ORGANIC SUBSTANCES, 3rd Year 28 28+7.3 PETROCHEMISTRY AND CARBOCHEMISTRY 4th Year 25+4.9 27 Language of instruction: HUNGARIAN IV. GRADUATION DIPLOMA 1 8 July Name of qualification: ENGINEER Test 1: Fundamental and speciality knowledge assesment 10 credits Official length of the programme of study : 8 semesters (4 years) Test 2: Defense of the graduation thesis 10 credits Mode of study: FULL TIME I. REQUIREMENTS FOR THE GRADUATION DIPLOMA V. THE ELECTION OF ELECTIVE SUBJECTS 240 credits from which: 1st Sem: A subject will be chosen from the Elective package 1 st foreign language 211 credits for the compulsory subjects; 2nd Sem: A subject will be chosen from the Elective package 2nd foreign language 29 credits for elective subjects; 6th Sem: A subject will be chosen from the Elective package 3 rd. and: 7th Sem: A subject will be chosen from the Elective package 4th. 21 credits graduation examination 8th Sem: For 5th Elective A subject will be chosen from the other faculties of BabeBolyai University. According to Law 1/2011 of the National Education Law, article 238, paragraph (1) A subject will be chosen from the Elective package 6th. -

Table of Contents
Table of Contents Introduction ................................. xi Chemical Glossary ............................. xvii Chapter 1. From the Chemical Element to Solids......... 1 1.1.CarbononEarth........................... 1 1.2.Abriefhistoryofthechemistryofcarbon.......... 5 1.2.1.Thefirstdiscoveries:fire,heatandmetals........ 9 1.2.2.Exploitationofminedresources.............. 11 1.2.3. Uses of dispersed carbons .................. 13 1.3. Presentation of carbon solids................... 14 1.3.1.Comparisonofnaturalandartificialevolution..... 16 1.3.2. Production and development of carbonaceous products ................................. 17 1.4.Conclusionandperspectives................... 18 1.5.Bibliography............................. 19 Chapter 2. The Polymorphism of Carbon.............. 23 2.1. The carbon atom and its chemical bonds ........... 24 2.1.1. Chemical bonds and solid phases ............. 24 2.1.2.Carbonisotopes ........................ 26 2.2.Athermodynamicapproach................... 27 2.2.1. Some reminders about phenomenonological thermodynamics ............................ 27 vi Carbon Science and Technology 2.2.2. Diagram of equilibrium states of carbon......... 28 2.3. New molecular phases ...................... 30 2.4. Non-crystalline carbons ..................... 32 2.4.1. Principal processes ...................... 33 2.4.2.Evolutionandstructuralcharacterizations ....... 35 2.4.3. Homogeneous massive carbons .............. 40 2.4.4. Porous and dispersed carbons ............... 42 2.5.Fromsolidstomaterials.................... -

Scientific Program
Scientific Program of the Fifth International Conference CATALYSIS FOR RENEWABLE SOURCES: FUEL, ENERGY, CHEMICALS CRS-5 Agios Nikolaos, Crete, Greece, September 2-6, 2019 Boreskov Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia Aristotle University of Thessaloniki, Greece http://conf.nsc.ru/CRS5 Conference Co-Chairs Professor Konstantinos Triantafyllidis Professor Vadim Yakovlev Aristotle University of Thessaloniki Boreskov Institute of Catalysis SB RAS Greece Russia Conference Proceedings: Special Issue CATALYSIS TODAY ELSEVIER CATALYSIS FOR SUSTAINABLE ENERGY (de Gruyter Open access) International Scientific Committee Chair: Laureate of the Global Energy Prize, Professor Valentin Parmon, Boreskov Institute of Catalysis SB RAS, Novosibirsk, Russia Professor Donato Aranda, Federal University of Rio de Janeiro, Brazil Professor Anthony Bridgwater, Aston University, United Kingdom Professor Jean-Claude Charpentier, Nancy University, France Professor Raghunath Vitthal Chaudhari, University of Kansas, Lawrence, USA Professor David Chiaramonti, University of Florence, Italy Professor Erik Heeres, University of Groningen, The Netherlands Professor Sascha Kersten, University of Twente, The Netherlands Professor Ivan Kozhevnikov, University of Liverpool, United Kingdom Professor Can Li, Dalian Institute of Chemical Physics, CAS, China Professor Guy Marin, Ghent University, Belgium Professor Simoni Plentz Meneghetti, Federal University of Alagoas, Brazil Professor Claude Mirodatos, Institute of Researchers on Catalysis and Environment in Lyon, France Professor Dmitry Murzin, Ǻbo Akademi University, Turku, Finland Professor Parasuraman Selvam, Indian Institute of Technology, Madras, India Professor Sergei Varfolomeev, Emanuel Institute of Biochemical Physics RAS, Moscow, Russia Program Committee Co-Chairs: Dr. Eleni Heracleous Dr. Maria Alekseeva Chemical Process & Energy Resources Institute Boreskov Institute of Catalysis SB RAS Thessaloniki, Greece Novosibirsk, Russia Program Committee Dr. -

Course: Chemistry
COURSE: CHEMISTRY DEGREE: BACHELOR DEGREE IN AEROSPACE ENGINEERING YEAR: 1st TERM: 2nd La asignatura tiene 29 sesiones que se distribuyen a lo largo de 14 semanas. Los laboratorios pueden situarse en cualquiera de ellas. Semanalmente el alumnos tendrá dos sesiones, excepto en un caso que serán tres. WEEKLY PLANNING Session in a different room room different a in Session Session with 2 professors professors 2 with Session GROUP WEEKLY PROGRAMMING FOR STUDENT (mark X) SESSION SESSION WEEK WEEK GENERAL DESCRIPTION SEMINARS SEMINARS LECTURES LECTURES MASTER MASTER GROUP GROUP CLASS/ CLASS/ HOMEWORK CLASS HOURS DESCRIPTION HOURS (Max. 7h per week) Basics of the atomic structure. Atomic number, mass number Introduction to the Course: General Course and isotopes. Introduction to the periodic table. Atoms, 1 1 Structure, Regulations and Grading. X NO 1.66 molecules and ions. The international system of units. Chemical TOPIC 0. Introduction to Chemistry 5 Equations. Stoichiometry. 1 2 TOPIC 0. Exercises on Topic 0 X NO Chemical Equations. Stoichiometry. 1.66 NO Basics of the Quantum theory. The periodic table. Periodic TOPIC 1. The Atomic Structure. Periodic 2 3 X properties of the elements. (Atomic Radius, Ionic Radius, 1.66 Properties Ionization Energy, Electron Affinity, Electronegativity) 7 TOPIC 1. Worked examples and Exercises in NO Practice on exercises in Electronic Structure and Periodic 2 4 X 1.66 class Properties of the elements. Página 1 de 4 NO Basic Concepts. The Ionic Bond. The Covalent Bond. Valence- Shell Electron-Pair Repulsion Models. Valence Bond Theory. 3 5 TOPIC 2. Chemical Bonding X 1.66 Hybridization of Atomic Orbitals. -

Technological Innovations
TECHNOLOGICAL INNOVATIONS FOR EZOLOGICALLY SUST'AlNABm DEWZIBPHENT.: The Case of the Chemical and Energy Irt- dust* in the Context of Energochemical Systems Technologies Development August 1987 WP-87-078 *Systems Research Department of the Institute for Control and Systems Engineering Academy of Mining and Metallurgy, Krakow **Industrial Chemistry Research lnstitute Department of Petro and Carbochemistry of the Industrial Chemistry Research Institute, Warsaw Working Papers are interim reports on work of the International Institute for Applied Systems Analysis and have received only Limited review. Views or opinions expressed herein do not necessarily represent those of the lnstitute or of its National Member Organizations. INTERNATIONAL INSTITUTE FOR APPLIED SYSTEMS ANALYSIS A-2361 Laxenburg, Austria FOREWORD The Integrated Energy Systems (IES) Project at IIASA has a wide range of colla- borative organizations in many countries involved in developing effective energy use and emission clean energy systems for the next century. This paper describes preliminary results of assessing the effects of integrating the energy and chemical industries in Poland. This is done on the basis of two coal and lignite conversion technologies (PYGAS and PYREG), taking into account specific economic and social conditions. Results obtained by the authors and presented in the paper show the good economic prospects for improving existing industrial systems by introducing the new coal technologies. VassiLi Okorokov Leader, Integrated Energy Systems This paper is aimed at the identification and evaluation of various development paths for the energy and chemical industries. This is to be embeded in the broader philosophy of the so-called Ecologically Sustainable Development (ESD). We focus our attention on the Polish energy and chemical industries since their development seems to be one of the key factors within the context of ESD. -
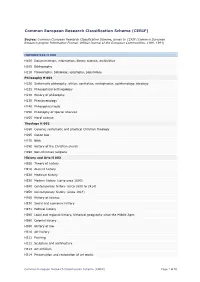
Common European Research Classification Scheme (CERIF)
Common European Research Classification Scheme (CERIF) Source: Common European Research Classification Scheme, annex to CERIF (Common European Research project Information Format, Official Journal of the European Communities, L189, 1991) HUMANITIES H 000 H100 Documentation, information, library science, archivistics H105 Bibliography H110 Paleography, bibliology, epigraphy, papyrology Philosophy H 001 H120 Systematic philosophy, ethics, aesthetics, metaphysics, epistemology, ideology H125 Philosophical anthropology H130 History of philosophy H135 Phenomenology H140 Philosophical logic H150 Philosophy of special sciences H155 Moral science Theology H 002 H160 General, systematic and practical Christian theology H165 Canon law H170 Bible H180 History of the Christian church H190 Non-Christian religions History and Arts H 003 H200 Theory of history H210 Ancient history H220 Medieval history H230 Modern history (up to circa 1800) H240 Contemporary history (circa 1800 to 1914) H250 Contemporary history (since 1914) H260 History of science H270 Social and economic history H271 Political history H280 Local and regional history, historical geography since the Middle Ages H290 Colonial history H300 History of law H310 Art history H311 Painting H312 Sculpture and architecture H313 Art criticism H314 Preservation and restoration of art works Common European Research Classification Scheme (CERIF) Page 1 of 10 H315 Aesthetics H320 Musicology H330 Dramatic art H340 Archaeology H341 Prehistory H345 Egyptology H346 Numismatics and sigillography H347 -

How to Cite Complete Issue More Information About This Article
DYNA ISSN: 0012-7353 ISSN: 2346-2183 Universidad Nacional de Colombia Meza-Fuentes, Edgardo; Rodriguez-Ruiz, Johana; Rangel, Maria do Carmo Characteristics of NiO present in solids obtained from hydrotalcites based on Ni/Al and Ni-Zn/Al DYNA, vol. 86, no. 210, 2019, July-September, pp. 58-65 Universidad Nacional de Colombia DOI: https://doi.org/10.15446/dyna.v86n210.78559 Available in: https://www.redalyc.org/articulo.oa?id=49662789007 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative Characteristics of NiO present in solids obtained from hydrotalcites based on Ni/Al and Ni-Zn/Al• Edgardo Meza-Fuentes a, Johana Rodriguez-Ruiz b & Maria do Carmo Rangel c a Grupo de Estudios en Materiales y Combustibles, Universidad de Cartagena, Cartagena, Colombia. [email protected] b Grupo de Investigación en Procesos de la Industria Petroquímica, SENA-Centro para la Industria Petroquímica, Cartagena, Colombia. [email protected] c Universidade Federal do Rio Grande do Sul, Porto Alegre, Brasil. [email protected] Received: March 19th, 2019. Received in revised form: May 2nd, 2019. Accepted: June 18th, 2019. Abstract NiO has a variety of applications, mainly in the production of electrochemical sensors and of metallic nickel. In addition, it is widely used as catalysts to produce hydrogen from natural gas. In this work, hydrotalcites based on nickel-aluminum and nickel-zinc-aluminum were synthesized, calcined at 500 °C and studied by different techniques.