Orientation of Pigeonite Exsolution Lamellae in Metamorphic Augite: Correlation with Composition and Calculated Optimal Phase Bo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SPECTRAL CHARACTERIZATION of the ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 and 8159. KJ Orr1, LV Forman1, GK Benedix1
Ninth International Conference on Mars 2019 (LPI Contrib. No. 2089) 6177.pdf SPECTRAL CHARACTERIZATION OF THE ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 AND 8159. K. J. Orr1, L. V. Forman1, G. K. Benedix1, M. J. Hackett2, V. E. Hamilton3, and A. R. Santos. 1Space Science and Technology Centre (SSTC), School of Earth and Planetary Sciences, Curtin University, Perth, Western Australia, Australia ([email protected]), 2School of Molecular and Life Sciences, Curtin University, Perth, Western Australia, Australia, 3Southwest Research Institute, 1050 Walnut St. #300, Boulder, CO 80302 USA. 4USRA, 7178 Columbia Gateway Dr., Columbia, MD 21046. Introduction: Thermal infrared (TIR) spectros- Ga), the oldest confirmed shergottites recovered so far copy is a powerful remote sensing tool used to unravel [5]. As the only shergottites of Early Amazonian to No- the surface compositions of a target body. This tech- achian in age, they provide an invaluable opportunity to nique has been widely used in space missions, because understanding Mars’ early history. of its ability to detect and determine modal mineralogy Methods: Both samples (~0.5g chips) were ac- of the surface geology. It has been instrumental in de- quired from UNM and were made into epoxy mounts. veloping our understanding of Mars, as the majority of NWA 8159 was analyzed with a Tescan Integrated Min- missions sent to Mars have included an infrared spec- eral Analyzer (TIMA) to determine modal mineral trometer. These spectrometers can operate either in the abundancies and produce high-resolution mineral maps. visible (VIS) to near-infrared (NIR) or in the mid-infra- NWA 8159 was also analyzed using EBSD to charac- red (MIR). -

Moore County Unbrecciated Cumulate Eucrite, 1.88Kg
Moore County Unbrecciated Cumulate Eucrite, 1.88 kg Seen to fall Figure 1a: The Moore County eucrite (fusion crust on left), from the collection of the North Carolina Museum of Natural Sciences. Scale from top to bottom of sample is ~ 5 cm. Photo courtesy of Chris Tacker. Introduction: The Moore County meteorite (Figures 1a,b,c) fell at 5:00 PM on April 21, 1913, on the farm of George C. Graves, located approximately three miles east of Carthage, Moore County, North Carolina (79o23’W, 35o25’N) (Henderson and Davis, 1936). A loud “rumbling and zooming” noise “with no distinct explosions” was first observed within a five or six mile radius of the fall, followed by a sighting of a red hot ball with a 15-foot trail of blue-black smoke; the meteorite itself landed within a few feet of a farmer, in a nearly-vertical (but slightly SW-sloping) hole in a freshly-plowed field (Henderson and Davis, 1936). Only one stone was recovered (Figure 1b), weighing approximately 1.88 kg (4 lbs. 2 oz.), with maximum dimensions approximately 15 cm x 10.5 cm x 8 cm (6 in x 4 3/16 in x 3 3/16 in) (Henderson and Davis, 1936). This stone was divided between the US National Museum (Smithsonian) in Washington, D.C., and the North Carolina State Museum in Raleigh, now the North Carolina Museum of Natural Sciences (Henderson and Davis, 1936), where the main fractions of the stone are still kept (0.9 kg at the USNM and ~0.56 kg at the NC Museum: Grady, 2000; Tacker, pers. -

The Investigated Rock Is One of the Gabbroic Bodies Which Crops out in the Surroundings of Lyngdal ( Extreme South of Norway)
PYROXENE RELATIONS IN A HYPERITE NEAR LYNGDAL, NORWAY JOHAN J. LAVREAU Lavreau, J. J.: Pyroxene relations in a hyperite near Lyngdal, Norway. Norsk Geologisk Tidsskrift, Vol. 50, pp. 333-340. Oslo 1970. Pyroxenes from a hyperite body associated with Precambrian gneisses were studied optically, chemically, and by means of X-rays. Pyroxene relations show that crystallization takes place at a temperature higher than the inversion of pigeonite, and proceeds into the stability field of hypersthene. The composition and the mutual relations between the pyroxenes suggest a crystallization process for these minerals. Johan J. Lavreau, Laboratoire de Mineralogie et Petrologie, Universite Libre de Bruxelles, Brussels, Belgium. Introduction The investigated rock is one of the gabbroic bodies which crops out in the surroundings of Lyngdal ( extreme South of Norway). It Iies 3 km north of the town, northwest of Skoland lake, and is crossed by the E 18 road to Flekkefjord (Fig. 1). It is a well defined and homogeneous crescent-shaped unit, about 1500 m long and 500 m broad. The rock is medium grained (1-2 mm grain size), dark grey in colour, and has a gabbro-dioritic compo sition (Niggli 1923, p. 126). Some dioritic varieties were also recognized (Table 1). The country rock is a monzonitic phenoblastic gneiss foliated parallel to the elongation of the hyperite body. The contacts are seldom visible, for the area is covered by recent deposits except in its northern part, where the rock is unfortunately tectonized and strongly retrometa morphosed. Experimental techniques Plagioclase and pyroxenes were first studied with the aid of the universal stage. -

Pigeonite Basalt 2109 Grams
12065 DRAFT Pigeonite Basalt 2109 grams Figure 1: Photo of 12065 showing numerous zap pits on rounded surface. Scale is in cm. NASA # S69-60591. Introduction Sample 12065 is a large rounded pigeonite basalt dated that near-surface olivine (Fo74) and some pyroxene at 3.16 ± 0.09 b.y. The outer surface is covered with settling could explain the variation in composition of micrometeorite pits on all sides (figure 1). some Apollo 12 basalts. Petrography Mineralogy 12065 is a variolitic basalt composed of pyroxene and Olivine: Olivine composition in 12065 ranges from olivine phenocrysts (figure 2) imbedded in a very fine Fo72-32 (Kushiro et al. 1971). matrix of feathery ilmenite, plagioclase and clinopyroxene (figure 3)(Reid 1971). Kushiro et al. Pyroxene: Hollister et al. (1971) and Kushiro et al. (1971) find that the fibrous pyroxene in 12065 is similar (1971) describe complex sector zoning of pyroxene to “quench pyroxenes” often found in quenching phenocrysts in 12065 (figure 4). Pigeonite cores are experiments. 12065 has a few percent void space. overgrown by subcalcic augite (Gay et al. 1971). Kushiro et al. report extreme Fe-enrichment in matrix Kushiro et al. (1971) used the bulk composition of pyroxene. Gay et al. report pyroxferroite with low Ca. 12065 to perform experiments leading to the conclusion Lunar Sample Compendium C Meyer 2005 Figure 2: Photomicrograph of thin section 12065 showing elongate pyroxene in variolitic groundmass. Scale is about 2 cm. NASA # S69-23378. Plagioclase: Plagioclase is An91 – An89 (Kushiro et al. 1971). Chemistry The chemical composition of 12065 has been reported Spinel: The Ti content of the Cr-spinel increases with by LSPET (1970), Maxwell et al. -

1 Hudson Valley Shakespeare Festival 2020 Community Bake-Off
Hudson Valley Shakespeare Festival 2020 Community Bake-Off Playwrighting Event Mahicantuck, The River That Flows Both Ways Sarah Johnson, Ph.D., Public History Consultant Hello, HVSF Bake-Off Playwrights! Here are some history resources to check out as inspiration for your plays. This year’s theme is rich in metaphors, as rivers and deltas have been used in literature, song, and the visual arts to portray life’s movements and confluences. In past years, I have directed participants to their local historical societies, museums, and libraries, to look at collections. This year, I’ve added more digital links because of the Covid-19 quarantine, so you can get inspiration from home, and can think more broadly and symbolically about Mahicantuck. Have a great time writing and thinking about Hudson’s River, as a 19th century map referred to it, and if you have the opportunity, go have a look at the Hudson River and let it inspire you in person. I look forward to the stories you will tell! 1909 Hudson-Fulton stamp The 1909 Fulton-Hudson Celebration was organized to commemorate Hudson’s 1609 “discovery” of the river. The stamp shows his ship the Half Moon, two Native American canoes, and Robert Fulton’s 1807 Clermont steamship on the Hudson. The official program here: https://library.si.edu/digital-library/book/officialprogram00huds also illustrated in the 1909 2 cent stamp and history: https://repository.si.edu/bitstream/handle/10088/8161/npm_1909_stamp.pdf and 2009 commemoration, https://www.themagazineantiques.com/article/the-hudson-fulton- celebration-100-years-later/ Geology & topography of the region: William J. -

2713^7 Contents
MINERALS OF WASHINGTON, D.C. AND VICINITY by Lawrence R. Bernstein U. S. Geo^r^'ce.l Survey OPEN F-'::. r;.".r'0.?;r cer.-..: 2713^7 CONTENTS Introduction 1 Scope of report 4 Mineral collecting 5 Acknowledgments 6 Introduction 6a ITT3 Aclj.il1 -> ! T^______.___~^. -"» _«_____«..«_»«__.. " " .._.«__._.._*_.__._.,_.._.-. _>-.-- -_>-->.-..-. Q Triassic deposits 31 Mineral localities 38 District of Columbia : 38 IMavtrlWlCfci JT XClilUl a Tirl ~ __ ___« - - -_ -»-i-___ .__- _ __- - ________________ m~m~m~ m~ m~ «M » M* **A^J ^ Anne Arundel County 43 Baltimore County 45 Howard County - 74 Montgomery County 88 Prince Georges County 120 Virginia . 129 Arlington County 129 Fairfax County 131 Fauquier County 139 Loudoun County 143 Prince William County 149 Diabase quarries of northern Virginia 155 CAPTIONS Illustrations Plate 1. Mineral localities of Washington, B.C., and vicinity. Plate 2. Generalized geologic map of Washington. D.C. and vicinity, Plate 3. Mineral deposits and generalized geology of the Triassic rocks near Washington, D.C. List of Figures Figure 1. Index map showing region covered in this report. Stfaded area is covered in most detail. Figure 2. Block diagram of the Washington, D.C. region showing physiographic provinces and major geographic and geologic features. ITfgure -3. Coastal Plain deposits of Washington, D.C. and vicinity. Figure 4. Generalized cross section of a typical complex pegmatite of the Washington, D.C. area. Figure 5. Rhythmically.layered gabbro of the Baltimore Gabbro Com plex at Ilchester, Maryland. Figure 6. Triassic diabase dike forming a ridge north of Route 7 near Dranesville, Virginia. -

Excerpts from the Book
Excerpts from Heaven Up-h’isted-ness! Copyright © 2011 by the Adirondack Forty-Sixers, Inc. All rights reserved. On the formation of the Forty-Sixers of Troy: During the early 1930s Bob Marshall’s booklet, “The High Peaks of the Adirondacks,” and Russell Carson’s Peaks and People of the Adirondacks captured the attention of a small group of outdoor enthusiasts from Grace Methodist Church in Troy, in particular the church’s pastor, the Rev. Ernest Ryder (#7), and two parishioners, Grace Hudowalski (#9) and Edward Hudowalski (#6)…. Ed and the Rev. Ryder had not, originally, intended to climb all 46. According to Ed, their goal was 25 peaks, but when they hit 27 “by accident,” they decided to climb 30. After reaching 30 they decided to climb all of them. The two finished arm-in-arm on Dix in the pouring rain on September 13, 1936. They shared a prayer of praise and thanks for their accomplishment. Less than six months after the Rev. Ryder and Ed finished their 46, the duo organized a club, comprised mainly of Ed Hudowalski’s Sunday School class, known as the Forty-Sixers of Troy. It was Ryder who coined the name “Forty-Sixer.” The term first appeared in print in an article in the Troy Record newspaper in 1937 announcing the formation of the hiking club: “Troy has its first mountain climbing club, all officers of which have climbed more than thirty of the major peaks in the Adirondacks. The club recently organized will be known as the Forty-sixers...” On Grace Hudowalski: Much like Bob Marshall, whose love of the wilderness was his all-consuming passion, Grace devoted her talents and energy, in both her professional and personal life, to promoting the exploration of New York State and in particular the Adirondack Mountains. -
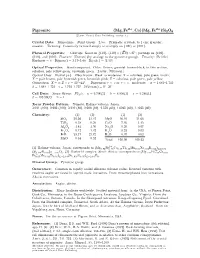
Pigeonite (Mg; Fe ; Ca)(Mg; Fe )Si2o6 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Monoclinic
2+ 2+ Pigeonite (Mg; Fe ; Ca)(Mg; Fe )Si2O6 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Prismatic crystals, to 1 cm; granular, massive. Twinning: Commonly twinned simply or multiply on 100 or 001 . f g f g Physical Properties: Cleavage: Good on 110 , (110) (110) 87 ; partings on 100 , f g ^ » ± f g 010 , and 001 . Fracture: [Uneven] (by analogy to the pyroxene group). Tenacity: [Brittle.] Hf ardgness =f6 Dg (meas.) = 3.17{3.46 D(calc.) = [3.53] Optical Properties: Semitransparent. Color: Brown, greenish brown-black; in thin section, colorless, pale yellow-green, brownish green. Luster: [Vitreous.] Optical Class: Biaxial (+). Pleochroism: Weak to moderate; X = colorless, pale green, brown; Y = pale brown, pale brownish green, brownish pink; Z = colorless, pale green, pale yellow. Orientation: X = a; Z c = 32 {44 . Dispersion: r < v or r > v; moderate. ® = 1.682{1.732 ^ ± ± ¯ = 1.684{1.732 ° = 1.705{1.757 2V(meas.) = 0±{30± Cell Data: Space Group: P 21=c: a = 9.706(2) b = 8.950(1) c = 5.246(1) ¯ = 108:59(1)± Z = 4 X-ray Powder Pattern: Yumoto, Hakone volcano, Japan. 3.021 (100), 2.903 (100), 3.210 (80), 2.908 (80), 2.578 (60), 1.6265 (60), 1.4935 (60) Chemistry: (1) (2) (1) (2) SiO2 50.56 51.47 MgO 16.10 21.68 TiO2 0.58 0.29 CaO 7.05 1.45 Al2O3 1.41 1.56 Na2O 0.26 0.07 Fe2O3 0.12 1.42 K2O 0.23 0.03 FeO 23.17 21.72 H2O¡ 0.07 0.02 MnO 0.54 0.52 Total 100.09 100.23 2+ (1) Hakone volcano, Japan; corresponds to (Mg0:92Fe0:73Ca0:29Ti0:02Mn0:02Na0:02K0:01)§=2:01 2+ (Si1:94Al0:06)§=2:00O6: (2) Bushveld complex, South Africa; corresponds to (Mg1:21Fe0:68Ca0:06 3+ Fe0:03Mn0:02Ti0:01)§=2:01(Si1:93Al0:07)§=2:00O6: Mineral Group: Pyroxene group. -

May-July 2008 No
MAY-JULY 2008 No. 0803 chepontuc — “Hard place to cross”, Iroquois reference to Glens Falls hepontuc ootnotes C T H E N E W S L E tt E R O F T H E G L E N S F ALLS- S ARAFT O G A C H A P T E R O F T H E A DIRO N DA C K M O U nt AI N C L U B Hikers alerted to muddy trails By Jim Schneider promote safety, hikers are advised to use Debar Mountain Wild Forest — trails only at lower elevations during the Azure Mountain New York State Department of spring mud season. Lower trails usually Giant Mountain Wilderness — Giant’s Environmental Conservation (DEC) urges are dry soon after snowmelt and are on less Washbowl and Roaring Brook Falls hikers of the Adirondack High Peaks to be erosive soils than the higher peaks. DEC is High Peaks Wilderness — Ampersand cautious during trips into the area and to asking hikers to avoid the following trails Mountain; Cascade; Big Slide; Brothers, postpone hiking on trails above 3,000 feet until muddy conditions have subsided: and Porter from Cascade; avoid all other until otherwise advised. High Peaks Wilderness Area — all trails approaches During warm and wet spring weather, above 3,000 feet—wet, muddy snow con- Hurricane Primitive Area — The many trails in higher and steeper por- ditions prevail, specifically at: Algonquin; Crows and Hurricane Mountain from tions of the Adirondacks can be become Colden; Feldspar; Gothics; Indian Pass; Route 9N hazardous to hikers. In the current muddy Lake Arnold Cross-Over; Marcy; Marcy McKenzie Mt. -

Crystallization History of Lunar F Eldspa Thic Basalt 14310
Crystallization History of Lunar F eldspa thic Basalt 14310 GEOLOGICAL SURVEY PROFESSIONAL PAPER 841 Prepared on behalf of the National Aeronautics and Space Administration Crystallization History of Lunar F eldspa thic Basalt 14 31 0 By ODETTE B. JAMES GEOLOGICAL SURVEY PROFESSIONAL PAPER 841 Prepared on behalf of the National Aeronautics and Space Administration An account of the crystallization history of an unusual type of lunar basalt, deduced from detailed petrographic studies and microprobe mineral analyses UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1973 UNITED STATES DEPARTMENT OF THE INTERIOR ROGERS C. B. MORTON, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress catalog-card No. 73-600177 For sale by the Superintendent of Documents U.S. Government Printing Office Washington, D.C. 20402- Price 70 cents domestic postpaid or 50 cents GPO Bookstore Stock Number 2401-00361 CONTENTS Page Page Abstract ---------------------------------------- 1 Phase compositions-Continued Introduction ------------------------------------ 1 Compositions of orthopyroxene and clinopyroxene 13 Mineral assemblage and texture ------------------ 2 at"their contacts -------------------------- Pigeonite and ferropig,eonite ------------------ 14 General features ---------------------------- 2 Augite _______ ------------------------------- 15 Locally developed fine-scale textures ---------- 3 Compositions of pigeonite and augite at their Orthopyroxene with preserved euhedral faces 4 contacts ---------------------------------- -

Ca–Mg Diffusion in Diopside: Tracer and Chemical Inter-Diffusion Coefficients
Contrib Mineral Petrol (2010) 159:175–186 DOI 10.1007/s00410-009-0422-5 ORIGINAL PAPER Ca–Mg diffusion in diopside: tracer and chemical inter-diffusion coefficients Xiaoyu Zhang Æ Jibamitra Ganguly Æ Motoo Ito Received: 20 March 2009 / Accepted: 8 July 2009 / Published online: 2 August 2009 Ó Springer-Verlag 2009 Abstract We have experimentally determined the tracer chemical diffusion coefficient of Ca and Mg must take into diffusion coefficients (D*) of 44Ca and 26Mg in a natural account the effect of thermodynamic factor (TF) on dif- diopside (*Di96) as function of crystallographic direction fusion coefficient. We calculate the dependence of the TF and temperature in the range of 950–1,150 °C at 1 bar and and the chemical interdiffusion coefficient, D(Ca–Mg), on f(O2) corresponding to those of the WI buffer. The exper- composition in the diopside–clinoenstatite mixture, using imental data parallel to the a*, b, and c crystallographic the available data on mixing property in this binary system. directions show significant diffusion anisotropy in the a–c Our D*(Ca) values parallel to the c axis are about 1–1.5 log and b–c planes, with the fastest diffusion being parallel units larger than those Dimanov et al. (1996). Incorporating to the c axis. With the exception of logD*(26Mg) parallel the effect of TF, the D(Ca–Mg) values calculated from our to the a* axis, the experimental data conform to the data at 1,100–1,200 °Cis*0.6–0.7 log unit greater than empirical diffusion ‘‘compensation relation’’, converging the experimental quasibinary D((Ca–Mg ? Fe)) data of to logD * -19.3 m2/s and T * 1,155 °C. -

Palladosilicide, Pd2si, a New Mineral from the Kapalagulu Intrusion, Western Tanzania and the Bushveld Complex, South Africa
Title Palladosilicide, Pd2Si, a new mineral from the Kapalagulu Intrusion, Western Tanzania and the Bushveld Complex, South Africa Authors Cabri, LJ; McDonald, AM; Rudashevsky, NS; Poirier, G; Wilhelmij, HR; Zhe, W; Rudashevsky, VN; Stanley, Christopher Date Submitted 2016-05-04 Palladosilicide, Pd2Si, a new mineral ……. 1 Palladosilicide, Pd2Si, a new mineral from the 2 Kapalagulu Intrusion, Western Tanzania and the 3 Bushveld Complex, South Africa 4 L. J. CABRI1*, A. M. MCDONALD2, C. J. STANLEY3, N. S. RUDASHEVSKY4, G. 5 POIRIER5, H.R. WILHELMIJ6, W. ZHE7, AND V.N. RUDASHEVSKY4 6 7 1Cabri Consulting Inc., 700-702 Bank Street, PO Box 14087, Ottawa, 8 Ontario, Canada K1S 3V0 9 2Department of Earth Sciences, Laurentian University, Ramsey Lake 10 Road, Sudbury, Ontario, Canada P3E 2C6 11 3Natural History Museum, Cromwell Road, London SW7 5BD, UK 12 4 CNT Instruments LTD, Svetlanovsky Ave. 75-41, St. Petersburg, 13 Russia 14 5Canadian Museum of Nature, Earth Science Research Services, 1740 15 Pink Road, Gatineau, Quebec J9J 3N7 (formerly Canmet, Ottawa) 16 6 Ore Deposit Geologist, 3 Norham End, North Oxford OX2 6SG, 17 England (formerly Lonmin-Goldstream JV, Australia) 18 7 Central Analytical Facility, Laurentian University, Ramsey Lake Road, 19 Sudbury, Ontario, Canada P3E 2C6 20 21 22 1 Palladosilicide, Pd2Si, a new mineral ……. 23 Abstract 24 Palladosilicide, Pd2Si, is a new mineral (IMA 2104-080) discovered in 25 chromite-rich samples from the Kapalagulu intrusion, western Tanzania 26 (30°03'51''E 5°53'16''S and 30°05'37''E 5°54'26''S) and from the UG-2 27 chromitite, Bushveld complex, South Africa.