Neural Oscillations and Event-Related Potentials Reveal How Semantic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
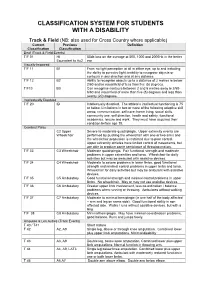
Disability Classification System
CLASSIFICATION SYSTEM FOR STUDENTS WITH A DISABILITY Track & Field (NB: also used for Cross Country where applicable) Current Previous Definition Classification Classification Deaf (Track & Field Events) T/F 01 HI 55db loss on the average at 500, 1000 and 2000Hz in the better Equivalent to Au2 ear Visually Impaired T/F 11 B1 From no light perception at all in either eye, up to and including the ability to perceive light; inability to recognise objects or contours in any direction and at any distance. T/F 12 B2 Ability to recognise objects up to a distance of 2 metres ie below 2/60 and/or visual field of less than five (5) degrees. T/F13 B3 Can recognise contours between 2 and 6 metres away ie 2/60- 6/60 and visual field of more than five (5) degrees and less than twenty (20) degrees. Intellectually Disabled T/F 20 ID Intellectually disabled. The athlete’s intellectual functioning is 75 or below. Limitations in two or more of the following adaptive skill areas; communication, self-care; home living, social skills, community use, self direction, health and safety, functional academics, leisure and work. They must have acquired their condition before age 18. Cerebral Palsy C2 Upper Severe to moderate quadriplegia. Upper extremity events are Wheelchair performed by pushing the wheelchair with one or two arms and the wheelchair propulsion is restricted due to poor control. Upper extremity athletes have limited control of movements, but are able to produce some semblance of throwing motion. T/F 33 C3 Wheelchair Moderate quadriplegia. Fair functional strength and moderate problems in upper extremities and torso. -

The Influence of Action Video Gaming Experience on the Perception of Emotional Faces and Emotional Word Meaning
Hindawi Neural Plasticity Volume 2021, Article ID 8841156, 12 pages https://doi.org/10.1155/2021/8841156 Research Article The Influence of Action Video Gaming Experience on the Perception of Emotional Faces and Emotional Word Meaning Yuening Yan ,1,2 Yi Li ,1,2 Xinyu Lou,1,2 Senqi Li,1,2 Yutong Yao,3 Diankun Gong ,1,2 Weiyi Ma ,4 and Guojian Yan 1,2 1The Clinical Hospital of Chengdu Brain Science Institute, MOE Key Lab for Neuroinformation, University of Electronic Science and Technology of China, Chengdu, China 2Center for Information in Medicine, School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu, China 3Faculty of Natural Science, University of Stirling, Stirling, UK 4School of Human Environmental Sciences, University of Arkansas, Fayetteville, AR, USA Correspondence should be addressed to Diankun Gong; [email protected], Weiyi Ma; [email protected], and Guojian Yan; [email protected] Received 31 August 2020; Revised 30 December 2020; Accepted 6 May 2021; Published 22 May 2021 Academic Editor: Jianzhong Su Copyright © 2021 Yuening Yan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Action video gaming (AVG) experience has been found related to sensorimotor and attentional development. However, the influence of AVG experience on the development of emotional perception skills is still unclear. Using behavioral and ERP measures, this study examined the relationship between AVG experience and the ability to decode emotional faces and emotional word meanings. -

Dolcos2020.Pdf
Neuroscience and Biobehavioral Reviews 108 (2020) 559–601 Contents lists available at ScienceDirect Neuroscience and Biobehavioral Reviews journal homepage: www.elsevier.com/locate/neubiorev Review article Neural correlates of emotion-attention interactions: From perception, T learning, and memory to social cognition, individual differences, and training interventions Florin Dolcosa,b,*, Yuta Katsumia,b, Matthew Moorea,b, Nick Berggrenc, Beatrice de Gelderd, Nazanin Derakshanc, Alfons O. Hamme, Ernst H.W. Kosterf, Cecile D. Ladouceurg, Hadas Okon-Singerh, Alan J. Pegnai, Thalia Richterh, Susanne Schweizerj, Jan Van den Stockk, Carlos Ventura-Bortl, Mathias Weymarl, Sanda Dolcosa,b,* a Department of Psychology, University of Illinois at Urbana-Champaign, Champaign, IL, USA b Beckman Institute for Advanced Science & Technology, University of Illinois at Urbana-Champaign, Urbana, IL, USA c Department of Psychological Sciences, Birkbeck, University of London, London, England, United Kingdom d Department of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands e Department of Biological and Clinical Psychology/Psychotherapy, University of Greifswald, Greifswald, Germany f Department of Experimental-Clinical and Health Psychology, Ghent University, Ghent, Belgium g Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, PA, USA h Department of Psychology, University of Haifa, Haifa, Israel i School of Psychology, University of Queensland, -

Developing a N400 Brain Computer Interface Based on Semantic Expectancy
Developing a N400 Brain Computer Interface based on semantic expectancy Francesco Chiossi 1, 2 1 Department of General Psychology, University of Padova, via Venezia 8, 35131 Padua, Italy 2 Neurotechnology Group, Technische Universität Berlin, Sekr. MAR 4-3, Marchstr 23, 10587 Berlin, Germany [email protected] Abstract. In this study, we present a new application to the study of the N400 related event component applied to the Brain Computer Interfaces (BCI) field. The N400 is classically defined in literature as an index of semantic integration mechanisms and it is sensitive to the difficulty with which the reader integrates the input within the semantic context, based on their expectations. By varying the level of violation of expectations in the semantic context and presenting sentences lacking the final word (cloze probability test) we want to train a classifier so that it can always complete the sentences in accordance with the expectations of the participant. The online classification is based on the av- erage peak differences in three different conditions (target, semantically related and unrelated), where the amplitude of the N400 should correlate with the pro- gressive and greater violation of semantic expectation. The findings can con- tribute significantly to this area of research that is still left with several unan- swered questions as this research is one of the first to exploit the N400 in an online experiment. Keywords: brain-computer interfacing, electroencephalography, N400, human- computer interaction, reading, expectancy, language, communication, seman- tics. 1 Introduction A brain-computer interface (BCI) provides a direct connection between the brain and an external device, translating brain signals into commands for electronic devices [1]. -

Electrophysiological Correlates of Anticipation and Emotional Memory
Durham E-Theses Electrophysiological correlates of anticipation and emotional memory TABASSUM, NAZOOL-E How to cite: TABASSUM, NAZOOL-E (2015) Electrophysiological correlates of anticipation and emotional memory, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/11560/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 Nazool -e Tabassum Electrophysiological correlates of anticipation and emotional memory Abstract This thesis investigated the role of anticipation as a mediating factor in the Emotion- Enhanced Memory (EEM) phenomenon. Using behavioural and ERP measures, three anticipatory conditions were explored: Informative, No-Cue and Non-Informative. The primary objective was to determine how far the pre-stimulus-Dm (Ps-Dm) effect is a reliable indicator of emotional memory encoding under different levels of anticipation, and if the preparatory process explanation accounts for any effects. This study also aimed to determine if there is an association between anticipatory activity at the pre- and post- stimulus phase, and the related behavioural outcome. -

Neurophysiological Processing of Architectural Ranking : Human Electrical Brain Responses to High- and Low-Ranking Buildings
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2011 Neurophysiological processing of architectural ranking : human electrical brain responses to high- and low-ranking buildings Oppenheim, Ilan Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-164054 Dissertation Published Version Originally published at: Oppenheim, Ilan. Neurophysiological processing of architectural ranking : human electrical brain re- sponses to high- and low-ranking buildings. 2011, University of Zurich, Faculty of Science. Neurophysiological Processing of Architectural Ranking Human Electrical Brain Responses to High- and Low-Ranking Buildings Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr. sc. nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Ilan Oppenheim von Maur, ZH Promotionskomitee: Prof. Dr. Lutz Jäncke (Vorsitz) Prof. Dr. Dr. med Thomas Grunwald (Leitung) Prof. Dr. Stephan Neuhauss (Fakultätsmitglied) Zürich, 2011 dedicated to Herr und Frau Braun I ACKNOWLEDGEMENTS II ACKNOWLEDGEMENTS ACKNOWLEDGEMENTS This doctoral thesis was carried out at the departments of Neurophysiology and Neuropsychology at the Swiss Epilepsy Centre in Zurich under the guidance of Prof. Dr. Dr. med Thomas Grunwald, head of department of Clinical Neurophysiology. I would like to express my sincere gratitude to the following: Prof. Dr. Dr. med Thomas Grunwald for sharing his immense experience and knowledge in all epilepsy and memory related questions, for his inspiration and support throughout my work, for his patience and critical reviews, and for tasteful musical discoveries. Prof. Dr. Hennric Jokeit for providing a colossal research office, for letting me be part of his team, and for tolerating my whistling. -

The N400 As an Index of Racial Stereotype Accessibility
Social Cognitive and Affective Neuroscience Advance Access published March 22, 2013 doi:10.1093/scan/nst018 SCAN (2013) 1 of 9 The N400 as an index of racial stereotype accessibility Eric Hehman,1 Hannah I. Volpert,2 and Robert F. Simons3 1Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH 03755, USA, 2Department of Psychological Sciences, University of Missouri, Columbia, MO 65211, USA, and 3Department of Psychology, University of Delaware, Newark, DE 19716, USA The current research examined the viability of the N400, an event-related potential (ERP) related to the detection of semantic incongruity, as an index of both stereotype accessibility and interracial prejudice. Participants EEG was recorded while they completed a sequential priming task, in which negative or positive, stereotypically black (African American) or white (Caucasian American) traits followed the presentation of either a black or white face acting as a prime. ERP examination focused on the N400, but additionally examined N100 and P200 reactivity. Replicating and extending previous N400 stereotype research, results indicated that the N400 can indeed function as an index of stereotype accessibility in an interracial domain, as greater N400 reactivity was elicited by trials in which the face prime was incongruent with the target trait than when primes and traits matched. Furthermore, N400 activity was moderated by participants self-reported explicit bias. More explicitly biased participants demonstrated greater N400 reactivity to stereotypically white traits following black faces than black traits following black faces. P200 activity was additionally associated with participants implicit biases, as more implicitly biased participants similarly demonstrated greater P200 reactivity to stereotypically white traits following black faces Downloaded from than black traits following black faces. -

N400 to Semantically Anomalous Pictures and Words
N400 to Semantically Anomalous Pictures and Words Arti Nigam, James E. Hoffian, and Robert F. Simons University of Delaware Abstract Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/4/1/15/1754910/jocn.1992.4.1.15.pdf by guest on 18 May 2021 The N400 component of the human event-related brain in one condition by a line drawing representing the same potential appears to be related to violations of semantic expec- concept (eg, the word “socks” was replaced by a picture of tancy during language comprehension.The present experiment socks). The N400 recorded in the Pictures Condition was found investigated whether the N400 is related specifically to activity to be identical to the N400 generated by words in terms of in a language system or is an index of a conceptual system that amplitude, scalp distribution, and latency. These results suggest is accessed by both pictures and words. Sentences were visually that the N400 is an index of activity in a conceptual memory presented one word at a time with the last word being replaced that is accessed by both pictures and words. INTRODUCTION 1985a) rather than in a sentence context. The principal finding is that pairs of unrelated words or words that do In a seminal study, Kutas and Hillyard (1980) discovered not fit into the category established by the other words a component of the human event-related brain potential in the series elicit N400s. This is presumably due to (ERP) that appeared to be uniquely associated with lan- priming by semantically related words and words be- guage processing. -

ERP Peaks Review 1 LINKING BRAINWAVES to the BRAIN
ERP Peaks Review 1 LINKING BRAINWAVES TO THE BRAIN: AN ERP PRIMER Alexandra P. Fonaryova Key, Guy O. Dove, and Mandy J. Maguire Psychological and Brain Sciences University of Louisville Louisville, Kentucky Short title: ERPs Peak Review. Key Words: ERP, peak, latency, brain activity source, electrophysiology. Please address all correspondence to: Alexandra P. Fonaryova Key, Ph.D. Department of Psychological and Brain Sciences 317 Life Sciences, University of Louisville Louisville, KY 40292-0001. [email protected] ERP Peaks Review 2 Linking Brainwaves To The Brain: An ERP Primer Alexandra Fonaryova Key, Guy O. Dove, and Mandy J. Maguire Abstract This paper reviews literature on the characteristics and possible interpretations of the event- related potential (ERP) peaks commonly identified in research. The description of each peak includes typical latencies, cortical distributions, and possible brain sources of observed activity as well as the evoking paradigms and underlying psychological processes. The review is intended to serve as a tutorial for general readers interested in neuropsychological research and a references source for researchers using ERP techniques. ERP Peaks Review 3 Linking Brainwaves To The Brain: An ERP Primer Alexandra P. Fonaryova Key, Guy O. Dove, and Mandy J. Maguire Over the latter portion of the past century recordings of brain electrical activity such as the continuous electroencephalogram (EEG) and the stimulus-relevant event-related potentials (ERPs) became frequent tools of choice for investigating the brain’s role in the cognitive processing in different populations. These electrophysiological recording techniques are generally non-invasive, relatively inexpensive, and do not require participants to provide a motor or verbal response. -

2019 NFCA Texas High School Leadoff Classic Main Bracket Results
2019 NFCA Texas High School Leadoff Classic Main Bracket Results Bryan College Station BHS CSHS 1 Bryan College Station 17 Thu 3pm Thu 3pm Cedar Creek BHS CSHS EP Eastlake SA Brandeis 49 Brandeis College Station 57 Cy Woods 1 BHS Thu 5pm Thu 5pm CSHS 2 18 Thu 1pm Brandeis Robinson Thu 1pm EP Montwood BHS CSHS Robinson 11 Huntsville 3 89 Klein Splendora 93 Splendora 9 BHS Fri 2pm Fri 2pm CSHS 3 Fredericksburg Splendora 19 Thu 9am Thu 9am Fredericksburg 6 VET1 VET2 Belton 6 Grapevine 50 58 Richmond Foster 11 BHS Thu 5pm Klein Splendora Thu 5pm CSHS 4 20 Thu 11am Klein Richmond Foster Thu 11am Klein 11 BHS CSHS Rockwall 2 SA Southwest 9 97 SA Southwest Cedar Ridge 99 Cy Ranch 7 VET1 Fri 4pm Fri 4pm CP3 5 Southwest Cy Ranch 21 Thu 11am Thu 1pm Temple 5 VET1 CP3 Plano East 5 Clear Springs 2 51 Southwest Alvin 59 Alvin VET1 Thu 3pm Thu 5pm CP3 6 22 Thu 9am Vandegrift Alvin Thu 3pm Vandegrift 5 BHS CSHS Lufkin San Marcos 5 90 94 Flower Mound 0 VET2 Fri 12pm Southwest Cedar Ridge Fri 12pm CP4 7 San Marcos Cedar Ridge 23 Thu 9am Thu 1pm Magnolia West 3 VET2 CHAMPIONSHIP CP4 RR Cedar Ridge 12 McKinney Boyd 3 52 Game 192 60 SA Johnson VET2 Thu 3pm San Marcos BHS Cedar Ridge Thu 5 pm CP4 8 4:00 PM 24 Thu 11am M. Boyd BHS 5 10 BHS Johnson Thu 3pm Manvel 0 129 Southwest vs. Cedar Ridge 130 Kingwood Park Bellaire 3 Sat 10am Sat 8am Deer Park VET4 BRAC-BB 9 Cedar Park Deer Park 25 Thu 11am Thu 1pm Cedar Park 5 VET4 BRAC-BB Leander Clements 13 53 Cedar Park MacArthur 61 SA MacArthur VET4 Thu 3pm Thu 5pm BRAC-BB 10 26 Thu 9am Clements MacArthur Thu 3pm Waco University 0 BHS CSHS Tomball Memorial Cy Fair 4 91 Friendswood Woodlands 95 Woodlands 2 VET5 Fri 8am Fri 8am BRAC-YS 11 San Benito Woodlands 27 Thu 11am Thu 1pm San Benito 10 VET5 BRAC-YS SA Holmes 1 Friendswood 10 54 62 Santa Fe VET5 Thu 3pm Friendswood Woodlands Thu 5pm BRAC-YS 12 28 Thu 9am Friendswood Santa Fe Thu 3pm Henderson 0 VET1 VET2 Lake Travis Ridge Point 16 98 100 B. -

Pdf/22/10/2289/1770340/Jocn.2009.21320.Pdf by Guest on 18 May 2021
Characterizing the Spatio-temporal Dynamics of the Neural Events Occurring prior to and up to Overt Recognition of Famous Faces Boutheina Jemel1, Anne-Marie Schuller2, and Valérie Goffaux3 Downloaded from http://mitprc.silverchair.com/jocn/article-pdf/22/10/2289/1770340/jocn.2009.21320.pdf by guest on 18 May 2021 Abstract ■ Although it is generally acknowledged that familiar face recog- of familiar faces. Although the N170 and the N250 face-sensitive nition is fast, mandatory, and proceeds outside conscious control, responses displayed an abrupt activity change at the moment of it is still unclear whether processes leading to familiar face recog- overt recognition of famous faces, later ERPs encompassing the nition occur in a linear (i.e., gradual) or a nonlinear (i.e., all-or- N400 and late positive component exhibited an incremental in- none) manner. To test these two alternative accounts, we recorded crease in amplitude as the point of recognition approached. In scalp ERPs while participants indicated whether they recognize addition, famous faces that were not overtly recognized at one as familiar the faces of famous and unfamiliar persons gradually trial before recognition elicited larger ERP potentials than unfamil- revealed in a descending sequence of frames, from the noisier to iar faces, probably reflecting a covert recognition process. Overall, the least noisy. This presentation procedure allowed us to charac- these findings present evidence that recognition of familiar faces terize the changes in scalp ERP responses occurring prior to and implicates spatio-temporally complex neural processes exhibit- up to overt recognition. Our main finding is that gradual and all- ing differential pattern activity changes as a function of recogni- or-none processes are possibly involved during overt recognition tion state. -

Classification Made Easy Class 1
Classification Made Easy Class 1 (CP1) The most severely disabled athletes belong to this classification. These athletes are dependent on a power wheelchair or assistance for mobility. They have severe limitation in both the arms and the legs and have very poor trunk control. Sports Available: • Race Runner (RR1) – using the Race Runner frame to run, track events include 100m, 200m and 400m. • Boccia o Boccia Class 1 (BC1) – players who fit into this category can throw the ball onto the court or a CP2 Lower who chooses to push the ball with the foot. Each BC1 athlete has a sport assistant on court with them. o Boccia Class 3 (BC3) – players who fit into this category cannot throw the ball onto the court and have no sustained grasp or release action. They will use a “chute” or “ramp” with the help from their sport assistant to propel the ball. They may use head or arm pointers to hold and release the ball. Players with a impairment of a non cerebral origin, severely affecting all four limbs, are included in this class. Class 2 (CP2) These athletes have poor strength or control all limbs but are able to propel a wheelchair. Some Class 2 athletes can walk but can never run functionally. The class 2 athletes can throw a ball but demonstrates poor grasp and release. Sports Available: • Race Runner (RR2) - using the Race Runner frame to run, track events include 100m, 200m and 400m. • Boccia o Boccia Class 2 (BC2) – players can throw the ball into the court consistently and do not need on court assistance.