Federal Register/Vol. 85, No. 221/Monday
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -

Schedule: November 16 – November 20 Antenna Channel 7.5 and WJCT More! Channel 7.4 (Comcast 212)
Schedule: November 16 – November 20 Antenna Channel 7.5 and WJCT More! Channel 7.4 (Comcast 212) CLICK ON THE LINKS FOR FREE ONLINE EDUCATIONAL RESOURCES MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY 16-Nov-20 17-Nov-20 18-Nov-20 19-Nov-20 20-Nov-20 Prehistoric Road Illustrative Math Gr 6 Prehistoric Road Illustrative Math Gr 6 Illustrative Math Gr 6 12:00PM Trip: Welcome to Trip: We Dig Unit 3, Lesson 1 Unit 6, Lesson 1 Unit 3, Lesson 4 Fossil Country Dinosaurs Illustrative Math Gr 7 Illustrative Math Gr 7 Illustrative Math Gr 7 12:30PM Unit 5, Lessons 3, 4 Unit 5, Lesson 5 Unit 2, Lesson 10 NOVA Illustrative Math Gr 8 NOVA Illustrative Math Gr 8 Illustrative Math Gr 8 1:00PM Making North America: Unit 5, Lesson 5 Making North Unit 5, Lesson 8 Unit 4, Lessons 13, 14 Origins America: Life Illustrative Math Alg Illustrative Math Alg Illustrative Math Alg 1:30PM Unit 6, Lesson 10 Unit 6, Lesson 11 Unit 6, Lesson 5 Power Trip Power Trip Power Trip Power Trip Poetry in America 2:00PM The Story of Energy: The Story of Energy: The Story of Energy: The Story of Energy: The New Colossus Water Food Transportation Wealth Poetry in America 2:30PM To Prisoners The African- Reconstruction: Reconstruction: Africa's Great American Masters 3:00PM Americans: Many America After the Civil America After the Civil Civilizations Sammy Davis, Jr.: I've Rivers to Cross War (Part 1, Hour 1) War, (Part 2, Hour 1) Origins Gotta Be Me 3:30PM The Black Atlantic (1500-1800) The African- Reconstruction: Reconstruction: Africa's Great 4:00PM Americans: Many America After the Civil America After the Civil Civilizations Rivers to Cross War (Part 1, Hour 2) War, (Part 2, Hour 2) The Cross and the The Age of Slavery Crescent 4:30PM (1800-1860) SCIENCE/MATH SOCIAL STUDIES ENGLISH/LANGUAGE ARTS Check out this viewing guide to take any show that you watch on PBS to the next level. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
November 16, 2020
November 16, 2020 Dr Pepper Hour Tuesday, November 17, 3-5 p.m. in the Barfield Drawing Room of the SUB. Since 1953, Dr Pepper Hour has been a long-standing tradition of the Student Union. This year we will be offering Dr Pepper Hour in a "Grab and Go" style to accommodate social distancing. Click HERE to learn about the Dr Pepper Hour Club! Don't miss the chance to earn your DP Hour Mug. Learn about the McNair Scholars Program! Tuesday, November 17, 6 p.m. on Zoom. The Baylor McNair Scholars Program aims to prepare first generation and other underrepresented students in acquiring the knowledge, skills and capacities necessary to successfully navigate a path to a Ph.D. program following graduation from Baylor. Program open to sophomores, juniors, and seniors! For more information, click HERE or contact [email protected]. Thanksgiving: The Native Perspective Wednesday, November 18, 2 p.m. at the Mayborn Museum. This year marks the 400th anniversary of the Pilgrims arriving in the New World, and while most Americans celebrate Thanksgiving as a day for rejoicing, Native peoples take a much different approach and call it a "National Day of Mourning" instead. Speaker: Dr. Julie Anne Sweet. For more information, click HERE or contact [email protected]. Thanksgiving Dinner Wednesday, November 18, 5-8 p.m. This year, Thanksgiving Dinner will be open to all students in a grab-n-go style. The meal will include turkey, stuffing, mashed potatoes, vegetable, and a dessert. On-campus, residential students can utilize walk-up access at any of the three dining halls (Penland, Memorial, or East Village.) Off-campus students should use the drive-thru option at Penland Hall to pick up your meal. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
![SCS TODAY | November 16, 2020 [A Late-Day M-W-F Bulletin from the SCS Dean’S Office]](https://docslib.b-cdn.net/cover/8516/scs-today-november-16-2020-a-late-day-m-w-f-bulletin-from-the-scs-dean-s-office-1778516.webp)
SCS TODAY | November 16, 2020 [A Late-Day M-W-F Bulletin from the SCS Dean’S Office]
SCS TODAY | November 16, 2020 [A late-day M-W-F bulletin from the SCS Dean’s Office] OF NOTE — Each year between November 13 – 19, people and organizations around the country participate in Transgender Awareness Week to help raise visibility about transgender people and address issues members of the community face. IMPORTANT REMINDER FOR RECORDING LECTURES — Faculty and staff can go to https://rooms.cs.cmu.edu and login using your Andrew ID. Search by building under SemiPrivate rooms for either Wean 3130-studio or GHC-4007-studio. Make one or more reservations. For additional guidance visit https://building.cs.cmu.edu/room-resources/howto.html ----------------------------------------------------------------------------------- On Mondays, SCS TODAY focuses on upcoming events that may be of interest to the SCS community. Mondays are always a good time to visit SCS EVENTS to see what’s on the calendar for the week. — Wednesday, November 18, 10 – 11:30 a.m.: 50 Years at CMU: The Inaugural Raj Reddy Artificial Intelligence Lecture. Be sure to register to attend this virtual event, and take a few minutes in advance to share a memory or offer congratulations to Raj Reddy on his 50+ years at CMU. Contact SCS Chief Advancement Officer Jenny Belardi for more details. — Wednesday – Friday, November 18 – 20: The Center for Informed Democracy and Social- cybersecurity (IDeaS) will host a virtual institute and conferencewhich includes invited panels, virtual posters, talks and tutorials. This event is free but registration is required. — Thursday, November 19, 6 – 7 p.m.: Bloomberg Engineering Tech Talk - Building Intelligent AI Agents for Game Worlds. -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -
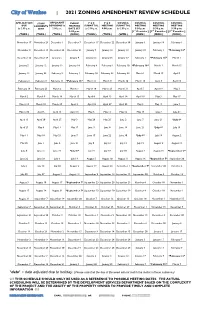
2021 Rezoning Review Schedule
City of Waukee | 2021 ZONING AMENDMENT REVIEW SCHEDULE APPLICATION STAFF APPLICANT PUBLIC P & Z P & Z COUNCIL COUNCIL COUNCIL COUNCIL DUE COMMENTS REVISIONS by HEARING SUBMITTAL MEETING SUBMITTAL MEETING MEETING MEETING by 5:00 p.m. SENT 5:00 p.m. DATE SET by 5:00 p.m. 6:00 p.m. by 5:00 p.m. 5:30 p.m. 5:30 p.m. 5:30 p.m. 5:30 p.m. [1st Consider.] [2nd Consider.] [3rd Consider.] (TUES.) (TUES.) (TUES.) (MON.) (THUR.) (TUES.) (WED.) (MON.) (MON.) (MON.) November 17 November 24 December 1 December 7 December 17 December 22 December 30 January 4 January 18 February 1 December 8 December 15 December 22 December 21 January 7 January 12 January 13 January 18 February 1 *February 16* December 22 December 29 January 5 January 4 January 21 January 26 January 27 February 1 *February 16* March 1 January 5 January 12 January 19 January 18 February 4 February 9 February 10 *February 16* March 1 March 15 January 19 January 26 February 2 February 1 February 18 February 23 February 24 March 1 March 15 April 5 February 2 February 9 February 16 *February 16* March 4 March 9 March 10 March 15 April 5 April 19 February 16 February 23 March 2 March 1 March 18 March 23 March 31 April 5 April 19 May 3 March 2 March 9 March 16 March 15 April 8 April 13 April 14 April 19 May 3 May 17 March 16 March 23 March 30 April 5 April 22 April 27 April 28 May 3 May 17 June 7 March 30 April 6 April 13 April 19 May 6 May 11 May 12 May 17 June 7 June 21 April 13 April 20 April 27 May 3 May 20 May 25 June 2 June 7 June 21 *July 6* April 27 May 4 May 11 May 17 June 3 June 8 June -

State Moves Alameda County to Purple Tier Starting at 12:01 A.M
FOR IMMEDIATE RELEASE Neetu Balram November 16, 2020 Public Information Manager Alameda County Public Health Department [email protected] State Moves Alameda County to Purple Tier Starting at 12:01 a.m. on November 18 previously open sectors and activities are restricted to State’s Purple Tier allowances and restrictions ALAMEDA COUNTY, CA – Due to very rapid and widespread increases in COVID-19 cases, the California Department of Public Health has modified its Blueprint for a Safer Economy to allow for a faster, more nimble response to the COVID-19 pandemic. Rather than using data from two weeks ago and moving only one tier at a time, the State is now using data from the past week and moving counties multiple tiers if necessary. These significant changes allow for a timelier response that will protect our communities, save lives, and keep our health care systems from becoming overwhelmed. Like other Bay Area counties, the Alameda County COVID-19 case rate continues to increase daily and hospitalizations are also on the rise. Today, the State placed Alameda County – along with 40 other counties – in the Purple Tier, the most restrictive level in the state's reopening framework. The State took this action in response to local and statewide increases in the daily COVID-19 case rates. What this means for Alameda County Businesses and Residents: • Effective at 12:01 a.m. on November 18, all activities and sectors must conform to the State’s requirements for counties in the Purple Tier. • Per the State’s requirements, any activity that was only permitted in the Red or Orange Tier will need to close, effective November 18. -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

Bulletin Publication Schedule for Calendar Year 2020
BULLETIN PUBLICATION SCHEDULE FOR CALENDAR YEAR 2020 Vol. Monthly Issue ARRF Due Closing Date for 21-day Comment Publication Date No. of Bulletin to DFM Agency Filing Period End Date 20-1 January 2020 November 15, 2019 *November 29, 2019 January 1, 2020 January 22, 2020 20-2 February 2020 December 20, 2019 January 3, 2020 February 5, 2020 February 26, 2020 20-3 March 2020 January 22, 2020 February 7, 2020 March 4, 2020 March 25, 2020 20-4 April 2020 February 21, 2020 March 6, 2020 April 1, 2020 April 22, 2020 20-5 May 2020 March 20, 2020 April 3, 2020 May 6, 2020 May 27, 2020 20-6 June 2020 April 24, 2020 May 1, 2020 June 3, 2020 June 24, 2020 20-7 July 2020 May 22, 2020 June 5, 2020 July 1, 2020 July 22, 2020 20-8 August 2020 June 19, 2020 July 3, 2020 August 5, 2020 August 26, 2020 20-9 September 2020 July 24, 2020 August 7, 2020 September 2, 2020 September 23, 2020 20-10 October 2020 August 21, 2020 **August 28, 2020 October 7, 2020 October 28, 2020 20-11 November 2020 September 18, 2020 October 2, 2020 November 4, 2020 November 25, 2020 20-12 December 2020 October 23, 2020 November 6, 2020 December 2, 2020 December 23, 2020 BULLETIN PUBLICATION SCHEDULE FOR CALENDAR YEAR 2021 Vol. Monthly Issue ARRF Due Closing Date for Bulletin 21-day Comment No. of Bulletin to DFM Agency Filing Publication Date Period End Date 21-1 January 2021 November 16, 2021 *November 30, 2020 January 6, 2021 January 27, 2021 21-2 February 2021 December 24, 2021 January 8, 2021 February 3, 2021 February 24, 2021 21-3 March 2021 January 22, 2021 February 5, 2021