ADHD) C Linical Edit Criteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Methylphenidate Amplifies the Potency and Reinforcing Effects Of
ARTICLE Received 1 Aug 2013 | Accepted 7 Oct 2013 | Published 5 Nov 2013 DOI: 10.1038/ncomms3720 Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression Erin S. Calipari1, Mark J. Ferris1, Ali Salahpour2, Marc G. Caron3 & Sara R. Jones1 Methylphenidate (MPH) is commonly diverted for recreational use, but the neurobiological consequences of exposure to MPH at high, abused doses are not well defined. Here we show that MPH self-administration in rats increases dopamine transporter (DAT) levels and enhances the potency of MPH and amphetamine on dopamine responses and drug-seeking behaviours, without altering cocaine effects. Genetic overexpression of the DAT in mice mimics these effects, confirming that MPH self-administration-induced increases in DAT levels are sufficient to induce the changes. Further, this work outlines a basic mechanism by which increases in DAT levels, regardless of how they occur, are capable of increasing the rewarding and reinforcing effects of select psychostimulant drugs, and suggests that indivi- duals with elevated DAT levels, such as ADHD sufferers, may be more susceptible to the addictive effects of amphetamine-like drugs. 1 Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston-Salem, North Carolina 27157, USA. 2 Department of Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada M5S1A8. 3 Department of Cell Biology, Medicine and Neurobiology, Duke University Medical Center, Durham, North Carolina 27710, USA. Correspondence and requests for materials should be addressed to S.R.J. (email: [email protected]). NATURE COMMUNICATIONS | 4:2720 | DOI: 10.1038/ncomms3720 | www.nature.com/naturecommunications 1 & 2013 Macmillan Publishers Limited. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Enhanced Reporting
Drug Profile, Targeted with Interpretation by Tandem Mass Spectrometry and Enzyme Immunoassay, Urine Patient: PAIN HYB 2, 2009288 | Date of Birth: | Gender: M | Physician: DR T. TEST Patient Identifiers: | Visit Number (FIN): Drug Analyte Result Cutoff Notes Meperidine metabolite Not Detected 50 ng/mL normeperidine Tapentadol Not Detected 100 ng/mL --Tapentadol-o-sulfate Not Detected 200 ng/mL tapentadol metabolite AMPHETAMINE-LIKE, MASS SPEC Amphetamine Present 50 ng/mL eg, Vyvanse; also a metabolite of methamphetamine Methamphetamine Present 200 ng/mL d- and l- isomers are not distinguished by this test; may reflect Vicks inhaler, Desoxyn, Selegiline, or illicit source MDMA - Ecstasy Not Detected 200 ng/mL MDA Not Detected 200 ng/mL also a metabolite of MDMA and MDEA MDEA - Eve Not Detected 200 ng/mL Phentermine Not Detected 100 ng/mL Methylphenidate Not Detected 100 ng/mL eg, Ritalin, Dexmethylphenidate, Focalin, Concerta BENZODIAZEPINE-LIKE, MASS SPEC Alprazolam Not Detected 40 ng/mL eg, Xanax --Alpha-hydroxyalprazolam Not Detected 20 ng/mL alprazolam metabolite Clonazepam Not Detected 20 ng/mL eg, Klonopin --7-aminoclonazepam Not Detected 40 ng/mL clonazepam metabolite Diazepam Not Detected 50 ng/mL eg, Valium Nordiazepam Not Detected 50 ng/mL metabolite of chlordiazepoxide (Librium), clorazepate (Tranxene), diazepam, halazepam (Alapryl), prazepam (Centrax) and others Oxazepam Not Detected 50 ng/mL eg, Serax; also metabolite of nordiazepam and temazepam Temazepam Not Detected 50 ng/mL eg, Restoril; also a metabolite of diazepam Lorazepam Not Detected 60 ng/mL eg, Ativan Midazolam Not Detected 20 ng/mL eg, Versed Zolpidem Present 20 ng/mL eg, Ambien Reference interval Creatinine value (mg/dL) 200.0 20.0 - 400.0 mg/dL Patient: PAIN HYB 2, 2009288 ARUP Accession: 21-048-107698 4848 Chart continues on following page(s) ARUP Enhanced Reporting | February 17, 2021 | page 4 of 5 Drug Profile, Targeted with Interpretation by Tandem Mass Spectrometry and Enzyme Immunoassay, Urine Patient: PAIN HYB 2, 2009288 | Date of Birth | Gender: M | Physician: DR T. -

The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines
The ICD-10 Classification of Mental and Behavioural Disorders: Clinical descriptions and diagnostic guidelines World Health Organization F10 - F19 Mental and behavioural disorders due to psychoactive substance use Overview of this block F10. – Mental and behavioural disorders due to use of alcohol F11. – Mental and behavioural disorders due to use of opioids F12. – Mental and behavioural disorders due to use of cannabinoids F13. – Mental and behavioural disorders due to use of sedative hypnotics F14. – Mental and behavioural disorders due to use of cocaine F15. – Mental and behavioural disorders due to use of other stimulants, including caffeine F16. – Mental and behavioural disorders due to use of hallucinogens F17. – Mental and behavioural disorders due to use of tobacco F18. – Mental and behavioural disorders due to use of volatile solvents F19. – Mental and behavioural disorders due to multiple drug use and use of other psychoactive substances Four- and five-character codes may be used to specify the clinical conditions, as follows: F1x.0 Acute intoxication .00 Uncomplicated .01 With trauma or other bodily injury .02 With other medical complications .03 With delirium .04 With perceptual distortions .05 With coma .06 With convulsions .07 Pathological intoxication F1x.1 Harmful use F1x.2 Dependence syndrome .20 Currently abstinent .21 Currently abstinent, but in a protected environment .22 Currently on a clinically supervised maintenance or replacement regime [controlled dependence] .23 Currently abstinent, but receiving treatment with -

Amphetamine/Dextroamphetamine IR Generic
GEORGIA MEDICAID FEE-FOR-SERVICE STIMULANT AND RELATED AGENTS PA SUMMARY Preferred Non-Preferred Amphetamine/dextroamphetamine IR generic Adzenys ER (amphetamine ER oral suspension) Armodafinil generic Adzenys XR (amphetamine ER dispersible tab) Atomoxetine generic Amphetamine/dextroamphetamine ER (generic Concerta (methylphenidate ER/SA) Adderall XR) Dextroamphetamine IR tablets generic Aptensio XR (methylphenidate ER) Focalin (dexmethylphenidate) Clonidine ER generic Focalin XR (dexmethylphenidate ER) Cotempla XR (methylphenidate ER disintegrating Guanfacine ER generic tablet) Methylin oral solution (methylphenidate) Daytrana (methylphenidate TD patch) Methylphenidate CD/CR/ER generic by Lannett Desoxyn (methamphetamine) [NDCs 00527-####-##] and Kremers Urban [NDCs Dexmethylphenidate IR generic 62175-####-##] (generic Metadate CD) Dexmethylphenidate ER generic Methylphenidate IR generic Dextroamphetamine ER capsules generic Modafinil generic Dextroamphetamine oral solution generic Quillichew ER (methylphenidate ER chew tabs) Dyanavel XR (amphetamine ER oral suspension) Quillivant XR (methylphenidate ER oral suspension) Evekeo (amphetamine tablets) Vyvanse (lisdexamfetamine) Methamphetamine generic Zenzedi 5 mg, 10 mg IR tablets (dextroamphetamine) Methylphenidate IR chewable tablets generic Methylphenidate ER/SA (generic Concerta) Methylphenidate ER/LA/SR (generic Ritalin LA, Ritalin SR, Metadate ER) Methylphenidate ER/SA 72 mg generic Methylphenidate oral solution generic Mydayis (amphetamine/dextroamphetamine ER) Ritalin LA 10 mg -

Current Topics in Behavioral Neurosciences
Current Topics in Behavioral Neurosciences Series Editors Mark A. Geyer, La Jolla, CA, USA Bart A. Ellenbroek, Wellington, New Zealand Charles A. Marsden, Nottingham, UK For further volumes: http://www.springer.com/series/7854 About this Series Current Topics in Behavioral Neurosciences provides critical and comprehensive discussions of the most significant areas of behavioral neuroscience research, written by leading international authorities. Each volume offers an informative and contemporary account of its subject, making it an unrivalled reference source. Titles in this series are available in both print and electronic formats. With the development of new methodologies for brain imaging, genetic and genomic analyses, molecular engineering of mutant animals, novel routes for drug delivery, and sophisticated cross-species behavioral assessments, it is now possible to study behavior relevant to psychiatric and neurological diseases and disorders on the physiological level. The Behavioral Neurosciences series focuses on ‘‘translational medicine’’ and cutting-edge technologies. Preclinical and clinical trials for the development of new diagostics and therapeutics as well as prevention efforts are covered whenever possible. Cameron S. Carter • Jeffrey W. Dalley Editors Brain Imaging in Behavioral Neuroscience 123 Editors Cameron S. Carter Jeffrey W. Dalley Imaging Research Center Department of Experimental Psychology Center for Neuroscience University of Cambridge University of California at Davis Downing Site Sacramento, CA 95817 Cambridge CB2 3EB USA UK ISSN 1866-3370 ISSN 1866-3389 (electronic) ISBN 978-3-642-28710-7 ISBN 978-3-642-28711-4 (eBook) DOI 10.1007/978-3-642-28711-4 Springer Heidelberg New York Dordrecht London Library of Congress Control Number: 2012938202 Ó Springer-Verlag Berlin Heidelberg 2012 This work is subject to copyright. -

Do You Know... Cocaine
Do You Know... Street names: blow, C, coke, crack, flake, freebase, rock, snow Cocaine What is cocaine? Cocaine is a stimulant drug. Stimulants make people feel more alert and energetic. Cocaine can also make people feel euphoric, or “high.” Pure cocaine was first isolated from the leaves of the coca bush in 1860. Researchers soon discovered that cocaine numbs whatever tissues it touches, leading to its use as a local anesthetic. Today, we mostly use synthetic anesthetics, rather than cocaine. In the 1880s, psychiatrist Sigmund Freud wrote scientific papers that praised cocaine as a treatment for many ailments, including depression and alcohol and opioid addiction. After this, cocaine became widely and legally available in patent medicines and soft drinks. As cocaine use increased, people began to discover its dangers. In 1911, Canada passed laws restricting the importation, manufacture, sale and possession of cocaine. The use of 1/4 © 2003, 2010 CAMH | www.camh.ca cocaine declined until the 1970s, when it became known How does cocaine make you feel? for its high cost, and for the rich and glamorous people How cocaine makes you feel depends on: who used it. Cheaper “crack” cocaine became available · how much you use in the 1980s. · how often and how long you use it · how you use it (by injection, orally, etc.) Where does cocaine come from? · your mood, expectation and environment Cocaine is extracted from the leaves of the Erythroxylum · your age (coca) bush, which grows on the slopes of the Andes · whether you have certain medical or psychiatric Mountains in South America. -

A Brief Overview of Psychiatric Pharmacotherapy
A Brief Overview of Psychiatric Pharmacotherapy Joel V. Oberstar, M.D. Chief Executive Officer Disclosures • Some medications discussed are not approved by the FDA for use in the population discussed/described. • Some medications discussed are not approved by the FDA for use in the manner discussed/described. • Co-Owner: – PrairieCare and PrairieCare Medical Group – Catch LLC Disclaimer The contents of this handout are for informational purposes only and are not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical or psychiatric condition. Never disregard professional/medical advice or delay in seeking it because of something you have read in this handout. Material in this handout may be copyrighted by the author or by third parties; reasonable efforts have been made to give attribution where appropriate. Caveat Regarding the Role of Medication… Neuroscience Overview Mind Over Matter, National Institute on Drug Abuse, National Institutes of Health. Available at: http://teens.drugabuse.gov/mom/index.asp. http://medicineworld.org/images/news-blogs/brain-700997.jpg Neuroscience Overview Mind Over Matter, National Institute on Drug Abuse, National Institutes of Health. Available at: http://teens.drugabuse.gov/mom/index.asp. Neurotransmitter Receptor Source: National Institute on Drug Abuse Common Diagnoses and Associated Medications • Psychotic Disorders – Antipsychotics • Bipolar Disorders – Mood Stabilizers, Antipsychotics, & Antidepressants • Depressive Disorders – Antidepressants • Anxiety Disorders – Antidepressants & Anxiolytics • Attention Deficit Hyperactivity Disorder – Stimulants, Antidepressants, 2-Adrenergic Agents, & Strattera Classes of Medications • Anti-depressants • Stimulants and non-stimulant alternatives • Anti-psychotics (a.k.a. -

Guide for Use and Monitoring of Psychotropic Medications in Children and Adolescents
Guide for Use and Monitoring of Psychotropic Medications In Children and Adolescents This document was developed by Community Care of North Carolina with the assistance of Fostering Health NC’s Medication Management Workgroup. The information contained in this guide is not intended to substitute or act as medical advice. If you have any questions about a medication prescribed to a child or adolescent in your care, contact the prescriber or a licensed medical professional. www.ncpeds.org/fosteringhealthnc 1 | P a g e Guide for Use and Monitoring of Psychotropic Medications In Children and Adolescents (Original 3.22.17 – revised 5.22.19) Definition of Psychotropic Medication: Medication used in the treatment of mental illnesses and capable of affecting the mind, emotions, and behavior. Use of this Information: The tables below offer information for child welfare workers, care managers and foster/resource parents to use when they want to learn more about a psychotropic medication. Specific information is outlined including therapeutic class, brand and generic names, FDA approved uses, common evidence-based uses, potential side effects, and medication-specific safety/effectiveness monitoring necessary when prescribed to a child or adolescent. Because few medications have been FDA approved for use in children 5 years of age and under, there is a column in the table that lists the FDA approved status and age ranges for the approved uses of each medication. This guide also provides a color-coded quick reference guide for each medication (Appendix A), questions that a foster/resource parent should ask a prescriber (Appendix B), and a glossary of terms (Appendix C). -
Pharmacy Prior Authorization Guideline 1. Criteria
Harvard Pilgrim Health Care – Pharmacy Prior Authorization Guideline Guideline Name ADHD Medications: Adderall (amphetamine-dextroamphetamine mixed salts), Adderall XR (amphetamine-dextroamphetamine mixed salts extended-release), Adzenys ER (amphetamine), Adzenys XR-ODT (amphetamine), Aptensio XR (methylphenidate), amphetamine, amphetamine-dextroamphetamine mixed salts, amphetamine-dextroamphetamine mixed salts extended-release, Concerta (methylphenidate), Cotempla-XR ODT (methylphenidate), Daytrana (methylphenidate), Desoxyn (methamphetamine), Dexedrine (dextroamphetamine), dexmethylphenidate, dexmethylphenidate extended- release, dextroamphetamine, dextroamphetamine extended-release, dextroamphetamine oral solution, Dyanavel XR (amphetamine), Evekeo (amphetamine), Focalin (dexmethylphenidate), Focalin XR (dexmethylphenidate), Jornay PM (methylphenidate), Metadate ER (methylphenidate), methamphetamine, Methylin oral solution (methylphenidate), methylphenidate, methylphenidate chewable tablets, methylphenidate extended-release, methylphenidate extended-release (CD), methylphenidate extended-release (LA), methylphenidate extended- release OSM, methylphenidate oral solution, Mydayis (amphetamine- dextroamphetamine), Procentra (dextroamphetamine), Quillivant XR (methylphenidate), Quillichew ER (methylphenidate), Relexxii (methylphenidate), Ritalin (methylphenidate), Ritalin LA (methylphenidate), Vyvanse (lisdexamfetamine), and Zenzedi (dextroamphetamine) 1. Criteria Product Name: Brand Adderall, Generic amphetamine-dextroamphetamine mixed -
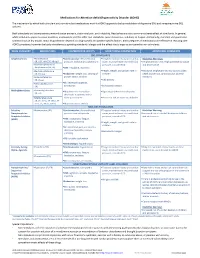
Medications for Attention Deficit/Hyperactivity Disorder (ADHD)
Medications for Attention-deficit/hyperactivity Disorder (ADHD) The mechanism by which both stimulant and non-stimulant medications work for ADHD appears to be by modulation of dopamine (DA) and norepinephrine (NE) systems. Both stimulants and atomoxetine commonly cause anorexia, abdominal pain, and irritability. Headaches are also a common adverse effect of stimulants. In general, while stimulants appear to cause insomnia, atomoxetine and the other non-stimulants cause drowsiness, sedation, or fatigue. Additionally, clonidine and guanfacine commonly cause dry mouth. Initial drug selection depends on drug-specific and patient-specific factors. Both categories of medications are effective in reducing core ADHD symptoms, however the body of evidence supporting stimulants is larger and the effect size is larger as compared to non-stimulants. DRUG CATEGORY MEDICATIONS COMMON SIDE EFFECTS MONITORING PARAMETERS ADDITIONAL COMMENTS CNS STIMULANTS Amphetamines .Amphetamine .Cardiovascular: elevated blood .Targeted cardiac history and cardiac Black Box Warnings: (IR, ODT, XR-ODT, XR-liq)* pressure, tachycardia, palpitations exam: monitor heart rate and blood .Amphetamines have a high potential for abuse .Dextroamphetamine/ pressure, consider ECG and dependence Amphetamine (IR, ER) .CNS: headache, insomnia .Dextroamphetamine .Height, weight, and growth rate in .Misuse of amphetamines may cause sudden (IR, ER, Liq) .Endocrine: weight loss, slowing of children death and serious cardiovascular adverse .Lisdexamfetamine growth rate in children reactions -

Download Thesis
This electronic thesis or dissertation has been downloaded from the King’s Research Portal at https://kclpure.kcl.ac.uk/portal/ The Role of Liquid Chromatography-High Resolution Mass Spectrometry in the Diagnosis and Treatment of Substance Misuse Murch, Sarah Louise Awarding institution: King's College London The copyright of this thesis rests with the author and no quotation from it or information derived from it may be published without proper acknowledgement. END USER LICENCE AGREEMENT Unless another licence is stated on the immediately following page this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence. https://creativecommons.org/licenses/by-nc-nd/4.0/ You are free to copy, distribute and transmit the work Under the following conditions: Attribution: You must attribute the work in the manner specified by the author (but not in any way that suggests that they endorse you or your use of the work). Non Commercial: You may not use this work for commercial purposes. No Derivative Works - You may not alter, transform, or build upon this work. Any of these conditions can be waived if you receive permission from the author. Your fair dealings and other rights are in no way affected by the above. Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 25. Sep. 2021 THE ROLE OF LIQUID CHROMATOGRAPHY- HIGH RESOLUTION MASS SPECTROMETRY IN THE DIAGNOSIS AND TREATMENT OF SUBSTANCE MISUSE Sarah Louise Murch (nee Belsey) Institute of Pharmaceutical Science King’s College London A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy Abstract Substance misuse remains problematic with current concerns being the rise in acute poisoning deaths, particularly opioid-associated, and the ever-widening range of drugs available.