Aquaculture Mapping Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dorset and East Devon Coast for Inclusion in the World Heritage List
Nomination of the Dorset and East Devon Coast for inclusion in the World Heritage List © Dorset County Council 2000 Dorset County Council, Devon County Council and the Dorset Coast Forum June 2000 Published by Dorset County Council on behalf of Dorset County Council, Devon County Council and the Dorset Coast Forum. Publication of this nomination has been supported by English Nature and the Countryside Agency, and has been advised by the Joint Nature Conservation Committee and the British Geological Survey. Maps reproduced from Ordnance Survey maps with the permission of the Controller of HMSO. © Crown Copyright. All rights reserved. Licence Number: LA 076 570. Maps and diagrams reproduced/derived from British Geological Survey material with the permission of the British Geological Survey. © NERC. All rights reserved. Permit Number: IPR/4-2. Design and production by Sillson Communications +44 (0)1929 552233. Cover: Duria antiquior (A more ancient Dorset) by Henry De la Beche, c. 1830. The first published reconstruction of a past environment, based on the Lower Jurassic rocks and fossils of the Dorset and East Devon Coast. © Dorset County Council 2000 In April 1999 the Government announced that the Dorset and East Devon Coast would be one of the twenty-five cultural and natural sites to be included on the United Kingdom’s new Tentative List of sites for future nomination for World Heritage status. Eighteen sites from the United Kingdom and its Overseas Territories have already been inscribed on the World Heritage List, although only two other natural sites within the UK, St Kilda and the Giant’s Causeway, have been granted this status to date. -

Web Site Dive Site List 28.12
OLD HARBOUR DIVE CENTRE DIVE SITES 2010 Aeolian Sky - DEPTH 30m This was a large ship some 16,000 tons that sank on 4th November 1979. Most of her hull is still intact with the holds still full of cargo. She lies on her port side with the decks facing east and her bows in the south. The bows themselves have been blown off and lie some 4 metres away from the main hull. Some salvage was carried out soon after her sinking, but most of her cargo still lies within her holds, most of it in unopened containers. Items of cargo still lay around the wreck on the sea bed. In good periods in summer, the visibility can be in excess of 15 metres. Alex Van Opstal - DEPTH 27m This Belgian passenger liner of 5,965 tons was the first of a series of large ships to be sunk by mines at the start of World War Two. The vessel was only two years old when she met her end on 15th September 1939. She is a large wreck and measured 420ft with a 57ft beam. Her forward emd is still intact and stands almost 7m off the sea bed. Towards the stern the ship is increasingly damaged. Her sertn section is broken of and located approxi- mately 100 yards to the south-west. Binnendijk - DEPTH 27m This 400ft Dutch steamship of almost 7,000 tons was sunk by a mine on 8th October 1939. The Binnendijk now rests on the seabed, badly broken up. Within the wreck’s lower parts there is coarse white sand. -

A Message from Tom Bromwich, Chairman Get A-Head Garden Party
COLOUR REFERENCES PANTONE 60% 2593 C:37 M:53 Y:0 K:0 WHITE C:0 M:0 Y:0 K:0 PANTONE 45% Process Black C:0 M:0 Y:0 K:45 NewsletterAutumn 2013 FONTS Q & A with Get A-Head’s new Trustee, Wendy Davies Avant GardeITC - Demi 1. How did you first get I was approached by John Watkinson, Hopefully, given my profession some involved with Get A-Head? my consultant and fellow trustee, to get financial sense!!! My first involvement was as involved. Having made a full recovery from 4. How would you describe yourself in A message from Tom Bromwich, Chairman five words? a patient making use of the throat cancer in 2009 and being helped Welcome to the Get A-Head Newsletter for I would also like to pay tribute to the Board of Trustees and in Sociable, friendly, hardworking, reliable and complimentary therapy that by the charity I felt it was my way of giving Autumn 2013, by the time you read this we particular the more recent members, Huw Griffiths, Shahz Ahmed, committed. the Charity offers. something back. will be in full swing with the organisation of Kristien Boelaert and Wendy Davies, all of whom bring invaluable 5. If you weren’t an Accountant, what this year’s Annual Ball, which promises to be help, support and knowledge, to ensure Get A-Head is progressing 2. Why did you decide that you wanted to 3. What do you think you will bring to would you be? a superb evening. We will also have had our in increasingly challenging times. -

Design Development - 3 Massing & Layout
Portland ERF Design and access statement Design development - 3 massing & layout 3434 Portland ERF Design and access statement 3535 Portland ERF Design and access statement DESIGN DEVELOPMENT - MASSING & LAYOUT 3.1 PUBLIC CONSULTATION 3.1.1 A public consultation was held at Portland Energy Recovery Facility What is Powerfuel Community Venue in Fortuneswell on Friday 5th Isle of Portland Portland proposing? December 2019. Powerfuel Portland is proposing to deliver an Energy Recovery Facility The Site The proposed new Energy Recovery Facility will process non-hazardous (ERF) at Portland Port in a £100 million investment. The site at Portland household and commercial waste which remains after recycling Port already has planning consent for an energy plant and Powerfuel The ERF that Powerfuel Portland is proposing activities, by using it as a fuel. The ERF uses effective, proven Portland is now preparing a new planning application to submit to provides a practical solution to a significant technology, with more than 40 energy from waste facilities operating in Dorset Council. local problem. The site is an existing the UK and many more across Europe. 3.1.2 At the event indicative proposals were brownfield site on industrial land owned by The facility will have the capacity to process 180,000 tonnes of refined RDF. Portland Port. This exhibition aims to provide information about our plans and answer RDF consists largely of combustible components of non-hazardous municipal included on the public consultation boards, as can be any questions that members of the public may have. The site already has planning consent for an solid waste, comprising mixed household, commercial and skip waste after the energy plant, which would have used either recyclable material has been removed. -

Technical Considerations
Portland ERF Design and access statement 8 Technical considerations 116116 Portland ERF Design and access statement 117117 CAPTION TITLE?????? Portland ERF Design and access statement TECHNICAL CONSIDERATIONS 8.1 INCLUSIVE DESIGN INTRODUCTION THE EQUALITY ACT (2010) AND DISABILITY 8.1.1 The design proposals have been developed with reference to regulations, adopted planning policy 8.1.5 The Equality Act has been in force since and guidance including: October 2010, and replaces, amongst other legislation, the Disability Discrimination Act. 7 • The Building Regulations 2010, Access to and use of buildings - Volume 2: Buildings other than 8.1.6 The Equality Act, although not prescriptive, dwellings, Approved Document M, HMSO, 2015 includes an intent to provide disabled people an • British Standard 8300:2009 +A1:2010 Design of accessible environment which does not discriminate buildings and their approaches to meet the needs against them because of their impairment. Statutory of disabled people - Code of practice, British regulations and recommendations for the built Standards Institution, 2010 environment provide parameters for how an accessible environment can be achieved. Compliance with these regulations and recommendations is not proof that 8 8.1.2 The design aspiration for this development Equality Act issues have been addressed. However, is the creation of an inclusive environment. All issues they do go a long way to ensuring such issues are 6 118118 relating to inclusive access have been, and will considered. continue to be, considered throughout the design process. 8.1.7 In the Act, the term disability includes not 5 2 only disabled people, but also people who have an 8.1.3 Our access strategy is based on an inclusive association with a disabled person (e.g. -

Portland Energy Recovery Facility
Portland energy recovery facility Design and access statement September 2020 2 6 2 7 0 1 b - T O R - X X - X X - R P - A - 0 0 0 3 Rev - Portland ERF Design and access statement 2 2 © Terence O’Rourke Ltd 2020. All rights reserved. No part of this document may be reproduced in any form or stored in a retrieval system without the prior written consent of the copyright holder. All figures (unless otherwise stated) © Terence O’Rourke Ltd 2020. Based upon the 2020 Ordnance Survey Mastermap vector data with the permission of the Ordnance Survey on behalf of Her Majesty’s Stationery Office, © Crown copy- right. Terence O’Rourke Ltd. Licence No. 100019980. Aerial imagery © Getmapping plc Portland ERF Design and access statement Contents Structure and content of the application Introduction Executive summary 11 The site and context 10 5 The final proposals 82 1.1 Site location 12 5.1 Massing principles 84 1.2 The site 14 5.2 Site layout 86 1.3 Visibility and sensitivities 16 5.3 Office building layout 88 1.4 Designations 18 5.4 Plant building layout 90 1.5 History and heritage 20 5.5 Concept elevational treatment 93 1.6 Planning history 24 5.6 Materials selection 94 1.7 Considerations and opportunities 26 5.7 Final proposals in context 97 22 Energy recovery facility 28 Access & movement, parking, deliveries and 6 100 2.1 The process & technical relationships 30 waste collection 2.2 Positioning the ERF plant on site 32 6.1 Deliveries and collections 102 6.2 Car access and parking 104 33 Design development - massing & layout 34 3 6.3 Pedestrian -

010 to Develop the Site for an Energy Plant Fuelled by Vegetable Oil, Including Waste Oils
NTS Portland energy recovery facility Environmental statement NTS Non-technical summary Portland Energy Recovery Facility (ERF) Powerfuel Portland Limited ES: Non-technical summary Non-technical summary Introduction NTS.1 Powerfuel Portland Limited is applying to Dorset Council for planning permission to develop land within Portland Port for an energy recovery facility (ERF) fuelled by refuse-derived fuel (RDF). Figure NTS.1 shows the application boundary and the location of the site in relation to Portland. NTS.2 An environmental impact assessment (EIA) was needed because of the potential for significant environmental effects. An environmental statement (ES) has been prepared to report the findings and its key elements are summarised in this non-technical summary. Background NTS.3 The former Weymouth and Portland Borough Council granted planning permission in early 2010 to develop the site for an energy plant fuelled by vegetable oil, including waste oils. This planning permission was varied in 2013 to allow waste rubber crumb from end-of-life tyres to be used as an alternative fuel source. NTS.4 The original plans were never completed and the development company that led the plans (W4B Portland Limited) no longer exists. Powerfuel Portland Limited is now bringing forward a new full planning application to develop the site for an ERF fuelled by RDF, which is a more standard and robust technology for the recovery of energy from waste. The proposed development site NTS.5 The 6.29-hectare(1) site lies on the north eastern coast of the Isle of Portland, within Portland Port, around 600 m east of the villages of Fortuneswell and Castletown (figure NTS.1). -
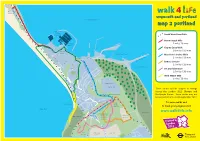
Weymouth Map 2: Portland
C H E S I L B E A C H P O R Chesil T L Beach A N Centre D B E A weymouth and portland C weymouth and portland H Portland Harbour R O A D A Mapmap 2 portland 3 5 4 National Sailing Academy South West Coast Path Hamm Beach Mile Portland 1 mile / 20 mins Marina H A M M B E Osprey Quay Walk AC H R P O 2.6 miles / 52 mins O A Osprey R D T L Quay A Portland Port N Merchant’s Incline Walk D B M E E 1.4 miles / 28 mins R Portland S TL E T O W N A E C A C S Castle H I D E Y E M Inner Breakwater R A V e Battery Circular W A O A E r D Y c D O R h O R 1.4 miles / 28 mins C E a D B n A L t Balaclava Bay O ’ U s R M I Art and Adventure E n L c T l Naval S i 1.9 miles / 38 mins n A e Cemetary C West Weare Mile D R Y 1 mile / 20 mins R O T V I C E V R C N E I H HM Prison N C I O C S M L W M I N O The Verne E E N L V R R E D O These routes will be subject to change L F N O T A R N D T O U R during the London 2012 Olympic and N R E D S Verne Local Nature Reserve W Paralympic Games. -

Ground Conditions and Water Quality Portland Energy Recovery Facility (ERF) ES Powerfuel Portland Limited Chapter 8: Ground Conditions and Water Quality
Portland energy recovery facility Environmental statement 8 8 Ground conditions and water quality Portland Energy Recovery Facility (ERF) ES Powerfuel Portland Limited Chapter 8: Ground conditions and water quality 8 Ground conditions and water quality Introduction 8.1 Arup was appointed to undertake the assessments of the potential for effects on ground conditions and water quality. Effects on flood risk were scoped out of the EIA and are addressed in the stand alone flood risk assessment submitted in support of the application. The findings of the assessments are summarised in this chapter and the full reports are included as technical appendices I1 (ground conditions) and I2 (water quality). The data sources and references used in the assessments are shown in table 8.1. British Geological Survey, 2000, 1:50,000 geological map series sheet 341 and part of 342, West Fleet and Weymouth. Solid and Drift British Standards Institute, 2019, BS 8485:2015+A1:2019 Code of practice for the design of protective measures for methane and carbon dioxide ground gases for new buildings British Standards Institute, 2017, BS 10175:2011+A2:2017 Investigation of potentially contaminated sites – code of practice Building Research Establishment, 2005, Special Digest 1: Concrete in aggressive ground, 3rd edition Building Research Establishment, 2004, Report 465: Cover systems for land regeneration: thickness of cover systems for contaminated land Building Research Establishment, 2003, Report 456: Control of dust from construction and demolition activities -
Proposed Portland Harbour Fishery Order Nature Conservation Review
Proposed Portland Harbour Fishery Order Nature Conservation Review Proposed Portland Harbour Fishery Order Nature Conservation Review version 7th August 2018 1. Introduction This technical note includes a review of potential impacts associated with Proposed Portland Harbour Fishery Order on Nature Conservation designations in and around Portland Harbour. 2. Relevant Documentation This includes: ➢ Portland Harbour Fishery Order ➢ Management plan ➢ Any documentation generated by third parties in connection with the above The need for a separate Water Framework Directive Risk Assessment would be assessed and undertaken in connection with any proposed activity associated with the Fishery Order and management Plan on a case-by-case basis. 3. Designated Sites Overview Figure 1 shows Portland Harbour Authority’s jurisdiction as defined by the Portland Harbour Revision Order. This is the same area that is subject to the application for the Portland Harbour Several Order. Designated European and national nature conservation sites and are shown and discussed in this report. Sites of Local Nature Conservation Importance are also shown but not discussed. The location of the proposal is sited within 2 km of the following: ➢ Studland to Portland Special Area of Conservation (SAC) ➢ Isle of Portland Site of Special Scientific Interest (SSSI) ➢ Isle of Portland to Studland Cliffs SAC ➢ Portland Harbour Shore SSSI 1 7th August 2018 Proposed Portland Harbour Fishery Order Nature Conservation Review ➢ Chesil & The Fleet SSSI ➢ Chesil Beach & The Fleet SPA ➢ Chesil Beach & The Fleet SAC ➢ Chesil Beach & The Fleet Wetland Area of International Importance under the Ramsar Convention (Ramsar site) ➢ Chesil Beach & Stennis Ledges Marine Conservation Zone (MCZ) Table 1 summarises designated sites adjacent or nearby to the harbour authority’s jurisdiction. -

Non-Technical Summary.Qxp
THE PROPOSED ISLE OF PORTLAND NATURAL GAS STORAGE FACILITY AND THE MAPPOWDER TO PORTLAND GAS PIPELINE PROJECT ENVIRONMENTAL STATEMENT NON-TECHNICAL SUMMARY 2 Figure 2.4.4.2: Cross-section through proposed cavern field Introduction 3 Portland Gas Storage Limited (Portland Gas) is seeking of thousands of new gas holders around the country for permission to build a natural gas storage facility on the the level of storage we will require by the middle of the Isle of Portland and for the other infrastructure necessary next decade. This is not a realistic solution today in to take and return gas to the National Grid's gas network. environmental or commercial terms. To obtain permission for the project Portland Gas must One of the safest and most environmentally friendly submit a total of seven applications, one to the method of storing large quantities of gas is deep Department of Trade and Industry for the pipeline and underground. A detailed understanding of the deep-lying a further six to Dorset County Council for the permanent geological formations resulting from deep drilling over 70 facilities and the temporary storage areas for the pipeline years or more has enabled us to target these formations. during construction. One such geological opportunity for storing gas is offered At a depth of 2,400 metres under Portland there is a very by deep-lying salt formations within which caverns can be thick layer of Triassic rock salt (halite), 220 million years created by dissolving ("leaching") the salt under controlled old. This salt can be dissolved safely and quickly using conditions to create voids into which gas can be stored. -

A Review of National and Local Sectoral Trends and Developments to Inform the C-SCOPE Marine Spatial Plan
A review of national and local sectoral trends and developments to inform the C-SCOPE Marine Spatial Plan Contents 1. Introduction 2 2.1 Dredging and Disposal 3 2.3 Cables and Pipelines 8 2.4 Oil and Gas 16 2.5 Offshore Renewables and Carbon Capture and Storage 20 2.6 Environmental Designations 28 2.7 Fisheries 32 2.8 Aquaculture 37 2.9 Marine Aggregates 44 2.10 Ports and Shipping 48 2.11 Tourism and Recreation 57 2.12 Coastal Defence 62 2.13 Ministry of Defence 65 1 1. Introduction Marine Spatial Planning is a future-oriented activity, and this document is an attempt to predict future sectoral development within the C-SCOPE Marine Management Area (MMA) (Figure 1). It draws on national and local trends to help inform policy development, and enables some cumulative effects to be considered. In addition to this document, planners are advised to consult Charting Progress 2, plus the C-SCOPE MMA and Socio-economic Reports which provide more in-depth information on national and local trends. Figure 1: C-SCOPE Marine Management Area. Source: C-SCOPE 2 2. Sector Assessments 2. 1 Dredging and Disposal 2.1.1 National Overview There are approximately 215 marine disposal sites for dredged material in the UK, but only half of these are in use at any one time. Since the 1980s there has been increasing legislation to prohibit the disposal of radioactive and industrial wastes, colliery minestone and sewage sludge. The disposal of material dredged from ports and harbours which cannot be used beneficially for other purposes is still permitted in licensed marine disposal sites.