Appendix D-5 Mojave Ground Squirrel Protocol Survey Results
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Delimiting Species in the Genus Otospermophilus (Rodentia: Sciuridae), Using Genetics, Ecology, and Morphology
bs_bs_banner Biological Journal of the Linnean Society, 2014, 113, 1136–1151. With 5 figures Delimiting species in the genus Otospermophilus (Rodentia: Sciuridae), using genetics, ecology, and morphology MARK A. PHUONG1*, MARISA C. W. LIM1, DANIEL R. WAIT1, KEVIN C. ROWE1,2 and CRAIG MORITZ1,3 1Department of Integrative Biology and Museum of Vertebrate Zoology, University of California, Berkeley, 3101 Valley Life Science Building, Berkeley, CA 94720, USA 2Sciences Department, Museum Victoria, Melbourne, VIC 3001, Australia 3Research School of Biology, The Australian National University, Acton, ACT 0200, Australia Received 16 April 2014; revised 6 July 2014; accepted for publication 7 July 2014 We apply an integrative taxonomy approach to delimit species of ground squirrels in the genus Otospermophilus because the diverse evolutionary histories of organisms shape the existence of taxonomic characters. Previous studies of mitochondrial DNA from this group recovered three divergent lineages within Otospermophilus beecheyi separated into northern, central, and southern geographical populations, with Otospermophilus atricapillus nested within the southern lineage of O. beecheyi. To further evaluate species boundaries within this complex, we collected additional genetic data (one mitochondrial locus, 11 microsatellite markers, and 11 nuclear loci), environmental data (eight bioclimatic variables), and morphological data (23 skull measurements). We used the maximum number of possible taxa (O. atricapillus, Northern O. beecheyi, Central O. beecheyi, and Southern O. beecheyi) as our operational taxonomic units (OTUs) and examined patterns of divergence between these OTUs. Phenotypic measures (both environmental and morphological) showed little differentiation among OTUs. By contrast, all genetic datasets supported the evolutionary independence of Northern O. beecheyi, although they were less consistent in their support for other OTUs as distinct species. -

Mammals of the California Desert
MAMMALS OF THE CALIFORNIA DESERT William F. Laudenslayer, Jr. Karen Boyer Buckingham Theodore A. Rado INTRODUCTION I ,+! The desert lands of southern California (Figure 1) support a rich variety of wildlife, of which mammals comprise an important element. Of the 19 living orders of mammals known in the world i- *- loday, nine are represented in the California desert15. Ninety-seven mammal species are known to t ':i he in this area. The southwestern United States has a larger number of mammal subspecies than my other continental area of comparable size (Hall 1981). This high degree of subspeciation, which f I;, ; leads to the development of new species, seems to be due to the great variation in topography, , , elevation, temperature, soils, and isolation caused by natural barriers. The order Rodentia may be k., 2:' , considered the most successful of the mammalian taxa in the desert; it is represented by 48 species Lc - occupying a wide variety of habitats. Bats comprise the second largest contingent of species. Of the 97 mammal species, 48 are found throughout the desert; the remaining 49 occur peripherally, with many restricted to the bordering mountain ranges or the Colorado River Valley. Four of the 97 I ?$ are non-native, having been introduced into the California desert. These are the Virginia opossum, ' >% Rocky Mountain mule deer, horse, and burro. Table 1 lists the desert mammals and their range 1 ;>?-axurrence as well as their current status of endangerment as determined by the U.S. fish and $' Wildlife Service (USWS 1989, 1990) and the California Department of Fish and Game (Calif. -

Special Publications Museum of Texas Tech University Number 63 18 September 2014
Special Publications Museum of Texas Tech University Number 63 18 September 2014 List of Recent Land Mammals of Mexico, 2014 José Ramírez-Pulido, Noé González-Ruiz, Alfred L. Gardner, and Joaquín Arroyo-Cabrales.0 Front cover: Image of the cover of Nova Plantarvm, Animalivm et Mineralivm Mexicanorvm Historia, by Francisci Hernández et al. (1651), which included the first list of the mammals found in Mexico. Cover image courtesy of the John Carter Brown Library at Brown University. SPECIAL PUBLICATIONS Museum of Texas Tech University Number 63 List of Recent Land Mammals of Mexico, 2014 JOSÉ RAMÍREZ-PULIDO, NOÉ GONZÁLEZ-RUIZ, ALFRED L. GARDNER, AND JOAQUÍN ARROYO-CABRALES Layout and Design: Lisa Bradley Cover Design: Image courtesy of the John Carter Brown Library at Brown University Production Editor: Lisa Bradley Copyright 2014, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (nsrl.ttu.edu). The authors and the Museum of Texas Tech University hereby grant permission to interested parties to download or print this publication for personal or educational (not for profit) use. Re-publication of any part of this paper in other works is not permitted without prior written permission of the Museum of Texas Tech University. This book was set in Times New Roman and printed on acid-free paper that meets the guidelines for per- manence and durability of the Committee on Production Guidelines for Book Longevity of the Council on Library Resources. Printed: 18 September 2014 Library of Congress Cataloging-in-Publication Data Special Publications of the Museum of Texas Tech University, Number 63 Series Editor: Robert J. -

Revised and Recirculated) Biological Resources
Section 4..2 (Revised and Recirculated) Biological Resources Note to reader: The County of San Bernardino has previously circulated for public review and comment the March 25, 2010, Draft Environmental Impact Report on the proposed Deep Creek project. Since the close of the public comment period for that DEIR, the County has made changes to Section 4.2, Biological Resources, of the DEIR. This revised Section 4.2 supersedes and replaces Section 4.2, Biological Resources, which was included in the March 25, 2010, DEIR. The County has elected to recirculate the revised Section 4.2 for public review and comment. Pursuant to Section 15088.5(c) of the CEQA Guidelines (California Code of Regulations, Title 14, Chapter 3), only Section 4.2 of the DEIR is being recirculated. Pursuant to Section 15088.5(f)(2) of the CEQA Guidelines, the County (i) is requesting that reviewers limit their comments to the revised Section 4.2, (ii) will respond to previously submitted comments on all portions of the previously circulated DEIR, except those made with respect to the now-superseded Section 4.2, and (iii) will respond to comments made during the recirculation period to the revised Section 4.2. Additionally, certain issues have previously been adjudicated, such as the evaluation and mitigation of potential impacts to the California desert tortoise and Mohave ground squirrel, and ultimately may be determined by the courts not to be subject to additional judicial review. 4.2 BIOLOGICAL RESOURCES This section evaluates the potential impacts on biological resources resulting from the implementation of the project. -
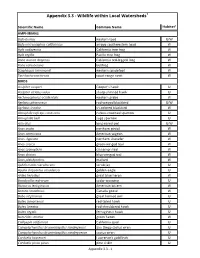
Appendix 3.3 - Wildlife Within Local Watersheds1
Appendix 3.3 - Wildlife within Local Watersheds1 2 Scientific Name Common Name Habitat AMPHIBIANS Bufo boreas western toad U/W Bufo microscaphus californicus arroyo southwestern toad W Hyla cadaverina California tree frog W Hyla regilla Pacific tree frog W Rana aurora draytonii California red-legged frog W Rana catesbeiana bullfrog W Scaphiopus hammondi western spadefoot W Taricha torosa torosa coast range newt W BIRDS Accipiter cooperi Cooper's hawk U Accipiter striatus velox sharp-shinned hawk U Aechmorphorus occidentalis western grebe W Agelaius phoeniceus red-winged blackbird U/W Agelaius tricolor tri-colored blackbird W Aimophila ruficeps canescens rufous-crowned sparrow U Aimophilia belli sage sparrow U Aiso otus long-eared owl U/W Anas acuta northern pintail W Anas americana American wigeon W Anas clypeata northern shoveler W Anas crecca green-winged teal W Anas cyanoptera cinnamon teal W Anas discors blue-winged teal W Anas platrhynchos mallard W Aphelocoma coerulescens scrub jay U Aquila chrysaetos canadensis golden eagle U Ardea herodius great blue heron W Bombycilla cedrorum cedar waxwing U Botaurus lentiginosus American bittern W Branta canadensis Canada goose W Bubo virginianus great horned owl U Buteo jamaicensis red-tailed hawk U Buteo lineatus red-shouldered hawk U Buteo regalis ferruginous hawk U Butorides striatus green heron W Callipepla californica California quail U Campylorhynchus brunneicapillus sandiegensis San Diego cactus wren U Campylorhynchus brunneicapillus sandiegoense cactus wren U Carduelis lawrencei Lawrence's -

Native Plants for Pollinators
Native Plants for a Pollinator Gardens Ginny Rosenkranz Extension Educator Commercial Horticulture [email protected] Why Native? • Consider that honeybees are NOT native! • Still…… Choose plants with pollen and nectar • Fragrant flowers • Composite flowers • Umbrella flowers Find the right color • Bees see Blue and Violet • Have a GREAT sense of smell • Like ‘landing pads’ • Tubular flowers Cover all the seasons • Spring flowers • Summer flowers • Fall flowers Include different shapes and sizes Plant in groups • Full sun • Protection from wind • Increases pollination Add water features Spring flowers • Phlox subulata - Moss Pink Spring flowers • Aquilegia – Columbine Spring flowers • Baptisia australis – False Indigo Spring flowers • Dicentra eximia – Fringed Bleeding Hearts Spring flowers • Geranium maculatum – Wild Geranium Spring flowers • Penstemon digitalis – Beard tongue Spring flowers • Salvia lyrata – Lyre leaf sage Spring flowers • Tradescantia virginiana - Spiderwort Viola sororia - Violet Summer annuals • Cleome hassleriana Annual summer flowers • Helianthus annuus - Sunflowers Annual summer flowers • Salvia Summer annuals • Tithonia rotundifolia – Mexican sunflower Summer annuals • Zinnia elegans Summer • Agastache anethiodora – Anise Hyssop Summer • Asclepias tuberosa – Butterfly weed Summer • Asclepias incarnate – swamp Milkweed Summer • Coreopsis lanceolata - Tickseed Summer • Coreopsis verticillata -Threadleaf Coreopsis Summer • Echinacea purpurea – Purple cone flower Summer • Eupatorium dubium- Joe Pye weed Summer • Filipendula -

45762554013.Pdf
Mastozoología Neotropical ISSN: 0327-9383 ISSN: 1666-0536 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Flores-Alta, Daniel; Rivera-Ortíz, Francisco A.; Contreras-González, Ana M. RECORD OF A POPULATION AND DESCRIPTION OF SOME ASPECTS OF THE LIFE HISTORY OF Notocitellus adocetus IN THE NORTH OF THE STATE OF GUERRERO, MEXICO Mastozoología Neotropical, vol. 26, no. 1, 2019, -June, pp. 175-181 Sociedad Argentina para el Estudio de los Mamíferos Argentina Available in: https://www.redalyc.org/articulo.oa?id=45762554013 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative Mastozoología Neotropical, 26(1):175-181, Mendoza, 2019 Copyright ©SAREM, 2019 Versión on-line ISSN 1666-0536 http://www.sarem.org.ar https://doi.org/10.31687/saremMN.19.26.1.0.02 http://www.sbmz.com.br Nota RECORD OF A POPULATION AND DESCRIPTION OF SOME ASPECTS OF THE LIFE HISTORY OF Notocitellus adocetus IN THE NORTH OF THE STATE OF GUERRERO, MEXICO Daniel Flores-Alta, Francisco A. Rivera-Ortíz and Ana M. Contreras-González Unidad de Biotecnología y Prototipos, Laboratorio de Ecología, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Los Reyes Iztacala. Tlalnepantla, Estado de México, México [correspondencia: Ana M. Contreras-González <[email protected]>]. ABSTRACT. The rodent Notocitellus adocetus is endemic to Mexico, and little is known about its life history. We describe a population of N. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Mammal Species Native to the USA and Canada for Which the MIL Has an Image (296) 31 July 2021
Mammal species native to the USA and Canada for which the MIL has an image (296) 31 July 2021 ARTIODACTYLA (includes CETACEA) (38) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei - Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Eschrichtius robustus - Gray Whale 7. Megaptera novaeangliae - Humpback Whale BOVIDAE - cattle, sheep, goats, and antelopes 1. Bos bison - American Bison 2. Oreamnos americanus - Mountain Goat 3. Ovibos moschatus - Muskox 4. Ovis canadensis - Bighorn Sheep 5. Ovis dalli - Thinhorn Sheep CERVIDAE - deer 1. Alces alces - Moose 2. Cervus canadensis - Wapiti (Elk) 3. Odocoileus hemionus - Mule Deer 4. Odocoileus virginianus - White-tailed Deer 5. Rangifer tarandus -Caribou DELPHINIDAE - ocean dolphins 1. Delphinus delphis - Common Dolphin 2. Globicephala macrorhynchus - Short-finned Pilot Whale 3. Grampus griseus - Risso's Dolphin 4. Lagenorhynchus albirostris - White-beaked Dolphin 5. Lissodelphis borealis - Northern Right-whale Dolphin 6. Orcinus orca - Killer Whale 7. Peponocephala electra - Melon-headed Whale 8. Pseudorca crassidens - False Killer Whale 9. Sagmatias obliquidens - Pacific White-sided Dolphin 10. Stenella coeruleoalba - Striped Dolphin 11. Stenella frontalis – Atlantic Spotted Dolphin 12. Steno bredanensis - Rough-toothed Dolphin 13. Tursiops truncatus - Common Bottlenose Dolphin MONODONTIDAE - narwhals, belugas 1. Delphinapterus leucas - Beluga 2. Monodon monoceros - Narwhal PHOCOENIDAE - porpoises 1. Phocoena phocoena - Harbor Porpoise 2. Phocoenoides dalli - Dall’s Porpoise PHYSETERIDAE - sperm whales Physeter macrocephalus – Sperm Whale TAYASSUIDAE - peccaries Dicotyles tajacu - Collared Peccary CARNIVORA (48) CANIDAE - dogs 1. Canis latrans - Coyote 2. -

GOOSEBERRYLEAF GLOBEMALLOW Sphaeralcea Grossulariifolia (Hook
GOOSEBERRYLEAF GLOBEMALLOW Sphaeralcea grossulariifolia (Hook. & Arn.) Rydb. Malvaceae – Mallow family Corey L. Gucker & Nancy L. Shaw | 2018 ORGANIZATION NOMENCLATURE Sphaeralcea grossulariifolia (Hook. & Arn.) Names, subtaxa, chromosome number(s), hybridization. Rydb., hereafter referred to as gooseberryleaf globemallow, belongs to the Malveae tribe of the Malvaceae or mallow family (Kearney 1935; La Duke 2016). Range, habitat, plant associations, elevation, soils. NRCS Plant Code. SPGR2 (USDA NRCS 2017). Subtaxa. The Flora of North America (La Duke 2016) does not recognize any varieties or Life form, morphology, distinguishing characteristics, reproduction. subspecies. Synonyms. Malvastrum coccineum (Nuttall) A. Gray var. grossulariifolium (Hooker & Arnott) Growth rate, successional status, disturbance ecology, importance to animals/people. Torrey, M. grossulariifolium (Hooker & Arnott) A. Gray, Sida grossulariifolia Hooker & Arnott, Sphaeralcea grossulariifolia subsp. pedata Current or potential uses in restoration. (Torrey ex A. Gray) Kearney, S. grossulariifolia var. pedata (Torrey ex A. Gray) Kearney, S. pedata Torrey ex A. Gray (La Duke 2016). Seed sourcing, wildland seed collection, seed cleaning, storage, Common Names. Gooseberryleaf globemallow, testing and marketing standards. current-leaf globemallow (La Duke 2016). Chromosome Number. Chromosome number is stable, 2n = 20, and plants are diploid (La Duke Recommendations/guidelines for producing seed. 2016). Hybridization. Hybridization occurs within the Sphaeralcea genus. -

L O U I S I a N A
L O U I S I A N A SPARROWS L O U I S I A N A SPARROWS Written by Bill Fontenot and Richard DeMay Photography by Greg Lavaty and Richard DeMay Designed and Illustrated by Diane K. Baker What is a Sparrow? Generally, sparrows are characterized as New World sparrows belong to the bird small, gray or brown-streaked, conical-billed family Emberizidae. Here in North America, birds that live on or near the ground. The sparrows are divided into 13 genera, which also cryptic blend of gray, white, black, and brown includes the towhees (genus Pipilo), longspurs hues which comprise a typical sparrow’s color (genus Calcarius), juncos (genus Junco), and pattern is the result of tens of thousands of Lark Bunting (genus Calamospiza) – all of sparrow generations living in grassland and which are technically sparrows. Emberizidae is brushland habitats. The triangular or cone- a large family, containing well over 300 species shaped bills inherent to most all sparrow species are perfectly adapted for a life of granivory – of crushing and husking seeds. “Of Louisiana’s 33 recorded sparrows, Sparrows possess well-developed claws on their toes, the evolutionary result of so much time spent on the ground, scratching for seeds only seven species breed here...” through leaf litter and other duff. Additionally, worldwide, 50 of which occur in the United most species incorporate a substantial amount States on a regular basis, and 33 of which have of insect, spider, snail, and other invertebrate been recorded for Louisiana. food items into their diets, especially during Of Louisiana’s 33 recorded sparrows, Opposite page: Bachman Sparrow the spring and summer months. -
![Germination and Seedling Establishment of Spiny Hopsage (Grayia Spinosa [Hook.] Moq.)](https://docslib.b-cdn.net/cover/8079/germination-and-seedling-establishment-of-spiny-hopsage-grayia-spinosa-hook-moq-328079.webp)
Germination and Seedling Establishment of Spiny Hopsage (Grayia Spinosa [Hook.] Moq.)
AN ABSTRACT OF THE THESIS OF Nancy L. Shaw for the degree of Doctor of Philosophy in Crop and Soil Sciences presented on March 19, 1992 Title: Germination and Seedling Establishment of Spiny Hopsage (Grayia Spinosa [Hook.] Moq.) Abstract approved:_Redactedfor Privacy von r. ULdUe Reestablishment of spiny hopsage(Grayia spinosa [Hook.] Moq.) where depleted or lost on shrub steppe sites can improve forage, plant cover, and soil stabilization. The objectives of this study were to: 1) determine direct-seeding requirements; 2) develop optimum germination pretreatments; and 3) examine dormancy mechanisms in spiny hopsage fruits and seeds. The effects of seed source, planting date,and site preparation method onseed germination and seedling establishment (SE) were examined at Birds of Prey and Reynolds Creek in southwestern Idaho. Three seed sources were planted on rough or compact seedbeds on 4 dates in 1986-87 and 3 dates in 1987-88. Exposure to cool-moist environments improved spring SE from early fall (EF) and late fall (LF) plantings. Few seedlings emerged from early (ESp) or late spring (LSp) plantings. SE was low at: 1 site in 1986-87 and atboth sites in 1987-88, probably due to lack of precipitation. For the successful 1986-87 planting, seedling density was greater on rough compared to compact seedbeds in April andMay, possiblydue to improved microclimate conditions. Growth rate varied among seed sources, but seedlings developed a deep taproot (mean length 266 mm) with few lateral roots the first season. Seeds were planted on 3 dates in 1986-87 and 1987-88, andnylon bags containing seeds were planted on 4 dates each year to study microenvironment effects on germination (G), germination rate (GR), and SE.