Genetic Structuring Among Colonies of a Pantropical Seabird: Implication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interim Accounts 3Rd Qtr 2017
STOLT-NIELSEN LIMITED UNAUDITED CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS For the Three and Nine Months Ended August 31, 2017 STOLT-NIELSEN LIMITED TABLE OF CONTENTS Condensed Consolidated Interim Income Statement for the Three and Nine Months Ended August 31, 2017 and 2016 3 Condensed Consolidated Interim Statement of Other Comprehensive Income for the Three and Nine Months Ended August 31, 2017 and 2016 4 Condensed Consolidated Interim Balance Sheet as of August 31, 2017 and November 30, 2016 5 Condensed Consolidated Interim Statement of Changes in Shareholders’ Equity for the Nine Months Ended August 31, 2017 and 2016 6 Condensed Consolidated Interim Statement of Cash Flows for the Nine Months Ended August 31, 2017 and 2016 7 Notes to the Condensed Consolidated Interim Financial Statements 8 Responsibility Statement 16 STOLT-NIELSEN LIMITED CONDENSED CONSOLIDATED INTERIM INCOME STATEMENT (UNAUDITED) Notes Three months ended Nine months ended August 31, August 31, August 31, August 31, 2017 2016 2017 2016 (in thousands, except per share data) Operating Revenue 4 $ 513,750 $ 474,069 $ 1,490,248 $ 1,416,910 Operating Expenses (342,569 ) (316,008 ) (990,182) (933,426) Gross Margin 171,181 158,061 500,066 483,484 Depreciation and amortization (66,828 ) (58,071 ) (195,924) (176,022) Gross Profit 104,353 99,990 304,142 307,462 Share of profit of joint ventures and associates 4,657 8,605 13,034 28,082 Administrative and general expenses (54,579 ) (52,709 ) (160,336) (153,557) Gain (loss) on disposal of assets, net 6 1,241 (1,999 -

156 Glossy Ibis
Text and images extracted from Marchant, S. & Higgins, P.J. (co-ordinating editors) 1990. Handbook of Australian, New Zealand & Antarctic Birds. Volume 1, Ratites to ducks; Part B, Australian pelican to ducks. Melbourne, Oxford University Press. Pages 953, 1071-1 078; plate 78. Reproduced with the permission of Bird life Australia and Jeff Davies. 953 Order CICONIIFORMES Medium-sized to huge, long-legged wading birds with well developed hallux or hind toe, and large bill. Variations in shape of bill used for recognition of sub-families. Despite long legs, walk rather than run and escape by flying. Five families of which three (Ardeidae, Ciconiidae, Threskiornithidae) represented in our region; others - Balaenicipitidae (Shoe-billed Stork) and Scopidae (Hammerhead) - monotypic and exclusively Ethiopian. Re lated to Phoenicopteriformes, which sometimes considered as belonging to same order, and, more distantly, to Anseriformes. Behavioural similarities suggest affinities also to Pelecaniformes (van Tets 1965; Meyerriecks 1966), but close relationship not supported by studies of egg-white proteins (Sibley & Ahlquist 1972). Suggested also, mainly on osteological and other anatomical characters, that Ardeidae should be placed in separate order from Ciconiidae and that Cathartidae (New World vultures) should be placed in same order as latter (Ligon 1967). REFERENCES Ligon, J.D. 1967. Occas. Pap. Mus. Zool. Univ. Mich. 651. Sibley, C. G., & J.E. Ahlquist. 1972. Bull. Peabody Mus. nat. Meyerriecks, A.J. 1966. Auk 83: 683-4. Hist. 39. van Tets, G.F. 1965. AOU orn. Monogr. 2. 1071 Family PLATALEIDAE ibises, spoonbills Medium-sized to large wading and terrestial birds. About 30 species in about 15 genera, divided into two sub families: ibises (Threskiornithinae) and spoonbills (Plataleinae); five species in three genera breeding in our region. -

Tube-Nosed Seabirds) Unique Characteristics
PELAGIC SEABIRDS OF THE CALIFORNIA CURRENT SYSTEM & CORDELL BANK NATIONAL MARINE SANCTUARY Written by Carol A. Keiper August, 2008 Cordell Bank National Marine Sanctuary protects an area of 529 square miles in one of the most productive offshore regions in North America. The sanctuary is located approximately 43 nautical miles northwest of the Golden Gate Bridge, and San Francisco California. The prominent feature of the Sanctuary is a submerged granite bank 4.5 miles wide and 9.5 miles long, which lay submerged 115 feet below the ocean’s surface. This unique undersea topography, in combination with the nutrient-rich ocean conditions created by the physical process of upwelling, produces a lush feeding ground. for countless invertebrates, fishes (over 180 species), marine mammals (over 25 species), and seabirds (over 60 species). The undersea oasis of the Cordell Bank and surrounding waters teems with life and provides food for hundreds of thousands of seabirds that travel from the Farallon Islands and the Point Reyes peninsula or have migrated thousands of miles from Alaska, Hawaii, Australia, New Zealand, and South America. Cordell Bank is also known as the albatross capital of the Northern Hemisphere because numerous species visit these waters. The US National Marine Sanctuaries are administered and managed by the National Oceanic and Atmospheric Administration (NOAA) who work with the public and other partners to balance human use and enjoyment with long-term conservation. There are four major orders of seabirds: 1) Sphenisciformes – penguins 2) *Procellariformes – albatross, fulmars, shearwaters, petrels 3) Pelecaniformes – pelicans, boobies, cormorants, frigate birds 4) *Charadriiformes - Gulls, Terns, & Alcids *Orders presented in this seminar In general, seabirds have life histories characterized by low productivity, delayed maturity, and relatively high adult survival. -

Download Statement
Stolt-Nielsen Limited c/o Stolt-Nielsen M.S. Ltd Tel: +44 20 7611 8960 65 Kingsway Fax: +44 20 7611 8965 London WC2B 6TD www.stolt-nielsen.com England Stolt-Nielsen Limited Reports Unaudited Results For the First Quarter of 2017 LONDON, April 6, 2017 – Stolt-Nielsen Limited (Oslo Børs: SNI) today reported unaudited results for the first quarter ended February 28, 2017. Net profit attributable to shareholders in the first quarter was $15.2 million, with revenue of $475.7 million, compared with a net profit of $22.8 million, with revenue of $463.0 million, in the fourth quarter of 2016. Highlights for the first quarter of 2017, compared with the fourth quarter of 2016, were: Stolt Tankers reported an operating profit of $28.5 million, down from $30.4 million, reflecting continued softness in the chemical tanker market, higher bunker fuel costs and a loss on a ship sold for early recycling, partially offset by the positive impact from the JO Tankers acquisition. The Stolt Tankers Joint Service Sailed-in Time-Charter Index was 0.68, down from 0.72. Stolthaven Terminals reported an operating profit of $16.7 million, up from $14.0 million, mainly reflecting improved results at Stolthaven Houston, Stolthaven Singapore and increased income from joint ventures. The prior quarter included gains of $2.3 million related to changes in the Company’s defined benefit pension and retiree healthcare plans. Stolt Tank Containers reported an operating profit of $9.0 million, down from $15.1 million, reflecting seasonally lower revenue, continued price competition and higher depreciation. -

Human Rights & Democracy the 2016 Foreign & Commonwealth Office
Human Rights & Democracy Cp 104 The 2018 Foreign & Commonwealth Office Report Human Rights & Democracy The 2018 Foreign & Commonwealth Office Report Presented to Parliament by the Secretary of State for Foreign and Commonwealth Affairs by Command of Her Majesty June 2019 Cp 104 Human Rights and Democracy: The 2018 Foreign & Commonwealth Office Report © Crown copyright 2019 This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. This publication is available at www.gov.uk/fco/human-rights-report Any enquiries regarding this publication should be sent to us at Communications Team, WH1.165, Multilateral Policy Directorate, Foreign and Commonwealth Office, King Charles Street, London SW1A 2AH ISBN 978-1-5286-1205-0 CCS0519142502 06/19 Printed on paper containing 75% recycled fibre content minimum Printed in the UK by the APS Group on behalf of the Controller of Her Majesty’s Stationery Office Designed in-house by the FCO Communication Directorate Cover image: Young Bangladeshi woman with candle (Getty Images) Human Rights and Democracy: The 2018 Foreign & Commonwealth Office Report Contents Preface by Foreign Secretary Jeremy Hunt ................................................................................. iv Foreword by Minister for Human Rights, Lord (Tariq) Ahmad of Wimbledon, the Prime Minister’s Special Envoy on Freedom of Religion or Belief, and Special Representative on Preventing Sexual Violence in Conflict ........................................................................................ v CHAPTER 1: Human Rights and Democracy Priority Themes ..................................................... -

Birds (DNA'dna Hybridization/Mtdna Sequences/Phylogeny/Systematics)
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 9861-9865, October 1994 Evolution Molecules vs. morphology in avian evolution: The case of the "pelecaniform" birds (DNA'DNA hybridization/mtDNA sequences/phylogeny/systematics) S. BLAIR HEDGES* AND CHARLES G. SIBLEyt *Department of Biology and Institute of Molecular Evolutionary Genetics, 208 Mueller Laboratory, Pennsylvania State University, University Park, PA 16802; and t433 Woodley Place, Santa Rosa, CA 95409 Contributed by Charles G. Sibley, June 20, 1994 ABSTRACT The traditional avian Order Pelecaniformes the three front toes has evolved in groups with separate is composed of birds with all four toes connected by a web. This origins-e.g., ducks, gulls, flamingos, and albatrosses. Could "totipalmate" condition is found in ca. 66 living species: 8 the totipalmate condition, which occurs in fewer species, also pelicans (Pelecanus), 9 boobies and gannets (Sula, Papasula, have multiple origins? Sibley and Ahlquist (2) reviewed the Morus), ca. 37 cormorants (Phalacrocorax) , 4 anhingas or literature from 1758 to 1990. darters (Anhinga), 5 frigatebirds (Fregata), and 3 tropicbirds There have been many morphological studies of the pele (Phaethon). Several additional characters are shared by these caniforms; those of Lanham (3), Saiff(4), and Cracraft (5) are genera, and their monophyly has been assumed since the among the most recent. Lanham (3) recognized their diversity beginning of modern zoological nomenclature. Most ornithol but concluded that the totipalmate birds form a natural order. ogists classify these genera as an order, although tropicbirds He assigned Phaethon and Fregata to separate suborders, have been viewed as related to terns, and frigatebirds as the other genera to the suborder Pelecani, and suggested that relatives of the petrels and albatrosses. -

2020 National Bird List
2020 NATIONAL BIRD LIST See General Rules, Eye Protection & other Policies on www.soinc.org as they apply to every event. Kingdom – ANIMALIA Great Blue Heron Ardea herodias ORDER: Charadriiformes Phylum – CHORDATA Snowy Egret Egretta thula Lapwings and Plovers (Charadriidae) Green Heron American Golden-Plover Subphylum – VERTEBRATA Black-crowned Night-heron Killdeer Charadrius vociferus Class - AVES Ibises and Spoonbills Oystercatchers (Haematopodidae) Family Group (Family Name) (Threskiornithidae) American Oystercatcher Common Name [Scientifc name Roseate Spoonbill Platalea ajaja Stilts and Avocets (Recurvirostridae) is in italics] Black-necked Stilt ORDER: Anseriformes ORDER: Suliformes American Avocet Recurvirostra Ducks, Geese, and Swans (Anatidae) Cormorants (Phalacrocoracidae) americana Black-bellied Whistling-duck Double-crested Cormorant Sandpipers, Phalaropes, and Allies Snow Goose Phalacrocorax auritus (Scolopacidae) Canada Goose Branta canadensis Darters (Anhingidae) Spotted Sandpiper Trumpeter Swan Anhinga Anhinga anhinga Ruddy Turnstone Wood Duck Aix sponsa Frigatebirds (Fregatidae) Dunlin Calidris alpina Mallard Anas platyrhynchos Magnifcent Frigatebird Wilson’s Snipe Northern Shoveler American Woodcock Scolopax minor Green-winged Teal ORDER: Ciconiiformes Gulls, Terns, and Skimmers (Laridae) Canvasback Deep-water Waders (Ciconiidae) Laughing Gull Hooded Merganser Wood Stork Ring-billed Gull Herring Gull Larus argentatus ORDER: Galliformes ORDER: Falconiformes Least Tern Sternula antillarum Partridges, Grouse, Turkeys, and -

2017 Annual Report 2016 - 2017
Annual Report Asheville School 2016 -2017 Annual Report 2016 - 2017 BOARD OF TRUSTEES (Fall 2017) An Education for an Inspired Life Mr. Walter G. Cox Jr. 1972, Chairman P ‘06 Published for Alumni & Friends of Asheville School Ms. Ann Craver, Co-Vice Chair P ‘11 by the Advancement Department Mr. Robert T. Gamble 1971, Co-Vice Chair Asheville School 360 Asheville School Road Mr. Marshall T. Bassett 1972, Treasurer Asheville, North Carolina 28806 828.254.6345 Dr. Audrey Alleyne P ’18, ’19 www.ashevilleschool.org (ex officio Parents’ Association) Editor Mr. Haywood Cochrane Jr. P ’17 Bob Williams Mr. Thomas E. Cone 1972 Assistant Head of School for Advancement Dan Seiden Mr. Matthew S. Crawford 1984 Mr. D. Tadley DeBerry 1981 Writers Alex Hill Mr. James A. Fisher 1964 Travis Price Dr. José A. González 1985 P ’20 Proof Readers Tish Anderson Ms. Mary Robinson Hervig 2002 Travis Price Bob Williams Ms. Jean Graham Keller 1995 Printing Mr. Richard J. Kelly 1968 P ’20 Lane Press Mr. Nishant N. Mehta 1998 Photographers Blake Madden Mr. Archibald R. Montgomery IV Sheila Coppersmith Bob Williams (ex officio Head of School) Eric Frazier Dr. Gregory K. Morris 1972 Mr. J. Allen Nivens Jr. 1993 A special thanks to the 1923 Memorial (ex officio Alumni Association) Archives for providing the archival photographs in this edition. Ms. Lara Nolletti P ’19 Mr. Laurance D. Pless 1971 P ’09, P ’13 Mr. Oliver G. Prince Jr. 1971 P ’00 Asheville School Mission: To prepare Mr. Arthur H. Rogers III 1988 our students for college and for life and to provide an atmosphere in Mr. -

Notes on the Phylogeny of the Pelecaniformes
Vol.•94764] .I LaNaaM,Phylogeny o[ the Pelecani[ormes 65 NOTES ON THE PHYLOGENY OF THE PELECANIFORMES BY URLESS N. LANHAM CERTAINaspects of the phylogenyof the order Pelecaniformesare obscuredby the arrangementof its three living subordersin fi•e standardclassifications. The sequenceas givenby Wetmore (1940) and Peters (1931)is: PHAJ•THONTES(Tropic-birds), PELECANI (Peli- cans, Boobies, Cormorants, Snake-birds),and FREGATAE(Frigate- birds). The tropic-birdsand œrigate-birds,although widely divergent, showbasic structural similaritieswhich indicate both to be primitive members of the order, and which link the order with the Pro- cellariiformes. The fundamentalsimilarity of Phagthonand Fregatahas been rec- ognizedin the olderwork on the comparativeosteology of the group. Mivart (1878),from a studyof the axial skeleton,came to the con- clusionthat Phagthonand Fregatapossessed common characters which sharplydistinguished them from the other steganopodes,and, in fact, could find no charactersto unite them with the rest of the stegano- podousgenera to form a natural group. The generalizedcondition of the cervicalvertebrae of thesetwo genera,in contrastto the spe- cialization in the rest of the order, and certain primitive skull char- acters are largely self-evidentand may have escapedemphasis for this reason. Shuœeldt(1894) statesthat" . Steganopodesare more closelyconnected with the Tubinares than they are with the Longi- pennes." Murphy (1936) recognizesthe generalizedcharacter of the skull of Pha•'thon, and the affinity of the Pelecaniformeswith the Procellariiformes;these two facts, at least, are implicit in modern classifications. The more obvious skeletal characters common to Pha•'thon and Fregata,and commonalso to the procellariiforms,may be summa- rized as follows: ¾omerpresent; maxillopalatines forming two conspicuousseparate lobes on the palatal surfaceof skull near anterior end of palatines;occipital condyle well un- derneath skull, so that condyle is anterior to coronal crest. -
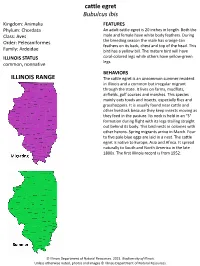
Cattle Egret Bubulcusibis ILLINOIS RANGE
cattle egret Bubulcus ibis Kingdom: Animalia FEATURES Phylum: Chordata An adult cattle egret is 20 inches in length. Both the Class: Aves male and female have white body feathers. During Order: Pelecaniformes the breeding season the male has orange-tan feathers on its back, chest and top of the head. This Family: Ardeidae bird has a yellow bill. The mature bird will have ILLINOIS STATUS coral-colored legs while others have yellow-green legs. common, nonnative BEHAVIORS ILLINOIS RANGE The cattle egret is an uncommon summer resident in Illinois and a common but irregular migrant through the state. It lives on farms, mudflats, airfields, golf courses and marshes. This species mainly eats toads and insects, especially flies and grasshoppers. It is usually found near cattle and other livestock because they keep insects moving as they feed in the pasture. Its neck is held in an "S" formation during flight with its legs trailing straight out behind its body. This bird nests in colonies with other herons. Spring migrants arrive in March. Four to five pale blue eggs are laid in a nest. The cattle egret is native to Europe, Asia and Africa. It spread naturally to South and North America in the late 1800s. The first Illinois record is from 1952. © Illinois Department of Natural Resources. 2021. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. adult male stevebyland/pond5.com © Illinois Department of Natural Resources. 2021. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. Aquatic Habitats lakes, ponds and reservoirs; temporary water supplies Woodland Habitats none Prairie and Edge Habitats edge © Illinois Department of Natural Resources. -

Fernald Preserve, Ohio Bird List
Bird List Order: ANSERIFORMES Order: PODICIPEDIFORMES Order: GRUIFORMES Order: COLUMBIFORMES Family: Anatidae Family: Podicipedidae Family: Rallidae Family: Columbidae Black-bellied Whistling Duck Pied-billed Grebe Virginia Rail Rock Pigeon Greater White-fronted Goose Horned Grebe Sora Mourning Dove Snow Goose Red-necked Grebe Common Gallinule Ross’s Goose Order: CUCULIFORMES American Coot Cackling Goose Order: SULIFORMES Family: Cuculidae Family: Gruidae Canada Goose Family: Phalacrocoracidae Yellow-billed Cuckoo Sandhill Crane Mute Swan Double-crested Cormorant Black-billed Cuckoo Trumpeter Swan Order: CHARADRIIFORMES Order: PELECANIFORMES Order: STRIGIFORMES Tundra Swan Family: Recurvirostridae Wood Duck Family: Ardeidae Family: Tytonidae Black-necked Stilt Gadwall American White Pelican Barn Owl Family: Charadriidae Eurasian Wigeon American Bittern Family: Strigidae Black-bellied Plover American Wigeon Least Bittern Eastern Screech-Owl American Golden Plover American Black Duck Great Blue Heron Great Horned Owl Semipalmated Plover Mallard Great Egret Barred Owl Killdeer Blue-winged Teal Snowy Egret Long-eared Owl Norther Shoveler Family: Scolopacidae Little Blue Heron Short-eared Owl Northern Pintail Spotted Sandpiper Cattle Egret Northern Saw-whet Owl Garganey Solitary Sandpiper Green Heron Greater Yellowlegs Green-winged Teal Order: CAPRIMULGIFORMES Black-crowned Night-Heron Canvasback Willet Family: Caprimulgidae Family: Threskiornithidae Redhead Lesser Yellowlegs Common Nighthawk Glossy Ibis Ring-necked Duck Upland Sandpiper White-faced -

A Story About Albatross
A Story About Albatross Tracking their Travels and Tracking Plastic Trash © Sophie Webb 2004 This is a story about tracking albatross and tracking plastic trash. In particular, this story is about the dark albatross (right side of image) – this is the Black-footed albatross. These birds criss-cross the entire Pacific Ocean like you and I criss-cross our backyards! 1 “If we didn’t clean our shorelines, where could the litter go?” “How can your coastal clean- up efforts benefit these unique birds?” These are two important questions to think about during this presentation. 2 Seabird Diversity H. Nevins J.Harvey Alcid WWW.nzbirds.com Penguin Pelican Petrel H. Nevins Four main orders of seabirds: Sphenisciformes - Penguins Procellariiformes – Albatrosses, Shearwaters, Fulmars, & Petrels Pelecaniformes - Pelicans, Cormorants, Boobies, Frigate birds Charadriiformes - Gulls, Terns, & Alcids There are 4 main orders of seabirds: Sphenisciformes, Procellariiformes, Pelecaniformes, and Charadriiformes. Seabirds that belong to the order Procellariiformes are among the most pelagic and far-ranging of seabirds that occur in all the oceans. This story is about seabirds from this group. 3 Seabird Feeding Methods FEEDERS Plunging (Ashmole 1971) Seabirds feed in three main ways: (1) they collect food items from the surface, (2) they plunge to capture submerged prey, and (3) they use their wings and feet to fly or swim underwater. The foraging methods of seabirds influence their ability to gather different types of prey and marine debris (e.g., floating versus sinking). Whereas most plastics float on the surface, other types may float deeper down in the water column. Thus, not all the debris that sinks at the surface ends up in the bottom of the sea.