Notes on the Phylogeny of the Pelecaniformes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

156 Glossy Ibis
Text and images extracted from Marchant, S. & Higgins, P.J. (co-ordinating editors) 1990. Handbook of Australian, New Zealand & Antarctic Birds. Volume 1, Ratites to ducks; Part B, Australian pelican to ducks. Melbourne, Oxford University Press. Pages 953, 1071-1 078; plate 78. Reproduced with the permission of Bird life Australia and Jeff Davies. 953 Order CICONIIFORMES Medium-sized to huge, long-legged wading birds with well developed hallux or hind toe, and large bill. Variations in shape of bill used for recognition of sub-families. Despite long legs, walk rather than run and escape by flying. Five families of which three (Ardeidae, Ciconiidae, Threskiornithidae) represented in our region; others - Balaenicipitidae (Shoe-billed Stork) and Scopidae (Hammerhead) - monotypic and exclusively Ethiopian. Re lated to Phoenicopteriformes, which sometimes considered as belonging to same order, and, more distantly, to Anseriformes. Behavioural similarities suggest affinities also to Pelecaniformes (van Tets 1965; Meyerriecks 1966), but close relationship not supported by studies of egg-white proteins (Sibley & Ahlquist 1972). Suggested also, mainly on osteological and other anatomical characters, that Ardeidae should be placed in separate order from Ciconiidae and that Cathartidae (New World vultures) should be placed in same order as latter (Ligon 1967). REFERENCES Ligon, J.D. 1967. Occas. Pap. Mus. Zool. Univ. Mich. 651. Sibley, C. G., & J.E. Ahlquist. 1972. Bull. Peabody Mus. nat. Meyerriecks, A.J. 1966. Auk 83: 683-4. Hist. 39. van Tets, G.F. 1965. AOU orn. Monogr. 2. 1071 Family PLATALEIDAE ibises, spoonbills Medium-sized to large wading and terrestial birds. About 30 species in about 15 genera, divided into two sub families: ibises (Threskiornithinae) and spoonbills (Plataleinae); five species in three genera breeding in our region. -

Tube-Nosed Seabirds) Unique Characteristics
PELAGIC SEABIRDS OF THE CALIFORNIA CURRENT SYSTEM & CORDELL BANK NATIONAL MARINE SANCTUARY Written by Carol A. Keiper August, 2008 Cordell Bank National Marine Sanctuary protects an area of 529 square miles in one of the most productive offshore regions in North America. The sanctuary is located approximately 43 nautical miles northwest of the Golden Gate Bridge, and San Francisco California. The prominent feature of the Sanctuary is a submerged granite bank 4.5 miles wide and 9.5 miles long, which lay submerged 115 feet below the ocean’s surface. This unique undersea topography, in combination with the nutrient-rich ocean conditions created by the physical process of upwelling, produces a lush feeding ground. for countless invertebrates, fishes (over 180 species), marine mammals (over 25 species), and seabirds (over 60 species). The undersea oasis of the Cordell Bank and surrounding waters teems with life and provides food for hundreds of thousands of seabirds that travel from the Farallon Islands and the Point Reyes peninsula or have migrated thousands of miles from Alaska, Hawaii, Australia, New Zealand, and South America. Cordell Bank is also known as the albatross capital of the Northern Hemisphere because numerous species visit these waters. The US National Marine Sanctuaries are administered and managed by the National Oceanic and Atmospheric Administration (NOAA) who work with the public and other partners to balance human use and enjoyment with long-term conservation. There are four major orders of seabirds: 1) Sphenisciformes – penguins 2) *Procellariformes – albatross, fulmars, shearwaters, petrels 3) Pelecaniformes – pelicans, boobies, cormorants, frigate birds 4) *Charadriiformes - Gulls, Terns, & Alcids *Orders presented in this seminar In general, seabirds have life histories characterized by low productivity, delayed maturity, and relatively high adult survival. -

Birds (DNA'dna Hybridization/Mtdna Sequences/Phylogeny/Systematics)
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 9861-9865, October 1994 Evolution Molecules vs. morphology in avian evolution: The case of the "pelecaniform" birds (DNA'DNA hybridization/mtDNA sequences/phylogeny/systematics) S. BLAIR HEDGES* AND CHARLES G. SIBLEyt *Department of Biology and Institute of Molecular Evolutionary Genetics, 208 Mueller Laboratory, Pennsylvania State University, University Park, PA 16802; and t433 Woodley Place, Santa Rosa, CA 95409 Contributed by Charles G. Sibley, June 20, 1994 ABSTRACT The traditional avian Order Pelecaniformes the three front toes has evolved in groups with separate is composed of birds with all four toes connected by a web. This origins-e.g., ducks, gulls, flamingos, and albatrosses. Could "totipalmate" condition is found in ca. 66 living species: 8 the totipalmate condition, which occurs in fewer species, also pelicans (Pelecanus), 9 boobies and gannets (Sula, Papasula, have multiple origins? Sibley and Ahlquist (2) reviewed the Morus), ca. 37 cormorants (Phalacrocorax) , 4 anhingas or literature from 1758 to 1990. darters (Anhinga), 5 frigatebirds (Fregata), and 3 tropicbirds There have been many morphological studies of the pele (Phaethon). Several additional characters are shared by these caniforms; those of Lanham (3), Saiff(4), and Cracraft (5) are genera, and their monophyly has been assumed since the among the most recent. Lanham (3) recognized their diversity beginning of modern zoological nomenclature. Most ornithol but concluded that the totipalmate birds form a natural order. ogists classify these genera as an order, although tropicbirds He assigned Phaethon and Fregata to separate suborders, have been viewed as related to terns, and frigatebirds as the other genera to the suborder Pelecani, and suggested that relatives of the petrels and albatrosses. -

2020 National Bird List
2020 NATIONAL BIRD LIST See General Rules, Eye Protection & other Policies on www.soinc.org as they apply to every event. Kingdom – ANIMALIA Great Blue Heron Ardea herodias ORDER: Charadriiformes Phylum – CHORDATA Snowy Egret Egretta thula Lapwings and Plovers (Charadriidae) Green Heron American Golden-Plover Subphylum – VERTEBRATA Black-crowned Night-heron Killdeer Charadrius vociferus Class - AVES Ibises and Spoonbills Oystercatchers (Haematopodidae) Family Group (Family Name) (Threskiornithidae) American Oystercatcher Common Name [Scientifc name Roseate Spoonbill Platalea ajaja Stilts and Avocets (Recurvirostridae) is in italics] Black-necked Stilt ORDER: Anseriformes ORDER: Suliformes American Avocet Recurvirostra Ducks, Geese, and Swans (Anatidae) Cormorants (Phalacrocoracidae) americana Black-bellied Whistling-duck Double-crested Cormorant Sandpipers, Phalaropes, and Allies Snow Goose Phalacrocorax auritus (Scolopacidae) Canada Goose Branta canadensis Darters (Anhingidae) Spotted Sandpiper Trumpeter Swan Anhinga Anhinga anhinga Ruddy Turnstone Wood Duck Aix sponsa Frigatebirds (Fregatidae) Dunlin Calidris alpina Mallard Anas platyrhynchos Magnifcent Frigatebird Wilson’s Snipe Northern Shoveler American Woodcock Scolopax minor Green-winged Teal ORDER: Ciconiiformes Gulls, Terns, and Skimmers (Laridae) Canvasback Deep-water Waders (Ciconiidae) Laughing Gull Hooded Merganser Wood Stork Ring-billed Gull Herring Gull Larus argentatus ORDER: Galliformes ORDER: Falconiformes Least Tern Sternula antillarum Partridges, Grouse, Turkeys, and -
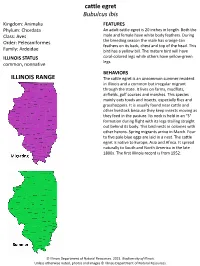
Cattle Egret Bubulcusibis ILLINOIS RANGE
cattle egret Bubulcus ibis Kingdom: Animalia FEATURES Phylum: Chordata An adult cattle egret is 20 inches in length. Both the Class: Aves male and female have white body feathers. During Order: Pelecaniformes the breeding season the male has orange-tan feathers on its back, chest and top of the head. This Family: Ardeidae bird has a yellow bill. The mature bird will have ILLINOIS STATUS coral-colored legs while others have yellow-green legs. common, nonnative BEHAVIORS ILLINOIS RANGE The cattle egret is an uncommon summer resident in Illinois and a common but irregular migrant through the state. It lives on farms, mudflats, airfields, golf courses and marshes. This species mainly eats toads and insects, especially flies and grasshoppers. It is usually found near cattle and other livestock because they keep insects moving as they feed in the pasture. Its neck is held in an "S" formation during flight with its legs trailing straight out behind its body. This bird nests in colonies with other herons. Spring migrants arrive in March. Four to five pale blue eggs are laid in a nest. The cattle egret is native to Europe, Asia and Africa. It spread naturally to South and North America in the late 1800s. The first Illinois record is from 1952. © Illinois Department of Natural Resources. 2021. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. adult male stevebyland/pond5.com © Illinois Department of Natural Resources. 2021. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. Aquatic Habitats lakes, ponds and reservoirs; temporary water supplies Woodland Habitats none Prairie and Edge Habitats edge © Illinois Department of Natural Resources. -

Fernald Preserve, Ohio Bird List
Bird List Order: ANSERIFORMES Order: PODICIPEDIFORMES Order: GRUIFORMES Order: COLUMBIFORMES Family: Anatidae Family: Podicipedidae Family: Rallidae Family: Columbidae Black-bellied Whistling Duck Pied-billed Grebe Virginia Rail Rock Pigeon Greater White-fronted Goose Horned Grebe Sora Mourning Dove Snow Goose Red-necked Grebe Common Gallinule Ross’s Goose Order: CUCULIFORMES American Coot Cackling Goose Order: SULIFORMES Family: Cuculidae Family: Gruidae Canada Goose Family: Phalacrocoracidae Yellow-billed Cuckoo Sandhill Crane Mute Swan Double-crested Cormorant Black-billed Cuckoo Trumpeter Swan Order: CHARADRIIFORMES Order: PELECANIFORMES Order: STRIGIFORMES Tundra Swan Family: Recurvirostridae Wood Duck Family: Ardeidae Family: Tytonidae Black-necked Stilt Gadwall American White Pelican Barn Owl Family: Charadriidae Eurasian Wigeon American Bittern Family: Strigidae Black-bellied Plover American Wigeon Least Bittern Eastern Screech-Owl American Golden Plover American Black Duck Great Blue Heron Great Horned Owl Semipalmated Plover Mallard Great Egret Barred Owl Killdeer Blue-winged Teal Snowy Egret Long-eared Owl Norther Shoveler Family: Scolopacidae Little Blue Heron Short-eared Owl Northern Pintail Spotted Sandpiper Cattle Egret Northern Saw-whet Owl Garganey Solitary Sandpiper Green Heron Greater Yellowlegs Green-winged Teal Order: CAPRIMULGIFORMES Black-crowned Night-Heron Canvasback Willet Family: Caprimulgidae Family: Threskiornithidae Redhead Lesser Yellowlegs Common Nighthawk Glossy Ibis Ring-necked Duck Upland Sandpiper White-faced -

A Story About Albatross
A Story About Albatross Tracking their Travels and Tracking Plastic Trash © Sophie Webb 2004 This is a story about tracking albatross and tracking plastic trash. In particular, this story is about the dark albatross (right side of image) – this is the Black-footed albatross. These birds criss-cross the entire Pacific Ocean like you and I criss-cross our backyards! 1 “If we didn’t clean our shorelines, where could the litter go?” “How can your coastal clean- up efforts benefit these unique birds?” These are two important questions to think about during this presentation. 2 Seabird Diversity H. Nevins J.Harvey Alcid WWW.nzbirds.com Penguin Pelican Petrel H. Nevins Four main orders of seabirds: Sphenisciformes - Penguins Procellariiformes – Albatrosses, Shearwaters, Fulmars, & Petrels Pelecaniformes - Pelicans, Cormorants, Boobies, Frigate birds Charadriiformes - Gulls, Terns, & Alcids There are 4 main orders of seabirds: Sphenisciformes, Procellariiformes, Pelecaniformes, and Charadriiformes. Seabirds that belong to the order Procellariiformes are among the most pelagic and far-ranging of seabirds that occur in all the oceans. This story is about seabirds from this group. 3 Seabird Feeding Methods FEEDERS Plunging (Ashmole 1971) Seabirds feed in three main ways: (1) they collect food items from the surface, (2) they plunge to capture submerged prey, and (3) they use their wings and feet to fly or swim underwater. The foraging methods of seabirds influence their ability to gather different types of prey and marine debris (e.g., floating versus sinking). Whereas most plastics float on the surface, other types may float deeper down in the water column. Thus, not all the debris that sinks at the surface ends up in the bottom of the sea. -

Diversity, Distribution and Habitat Association of Birds in Menze-Guassa Community Conservation Area, Central Ethiopia
Vol. 10(9), pp. 372-379, September 2018 DOI: 10.5897/IJBC2018.1196 Article Number: 883998C58352 ISSN: 2141-243X Copyright ©2018 International Journal of Biodiversity and Author(s) retain the copyright of this article http://www.academicjournals.org/IJBC Conservation Full Length Research Paper Diversity, distribution and habitat association of birds in Menze-Guassa Community Conservation Area, Central Ethiopia Yihenew Aynalem* and Bezawork Afework Department of Zoological Sciences, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia. Received 27 April, 2018; Accepted 14 June, 2018 A study was conducted in Menz-Guassa Community Conservation Area (MGCCA) from November 2016 to March 2017, to assess the diversity, distribution and habitat association of birds. Three habitat types including forest, grassland, and moorland habitats were identified based on their vegetation composition. Point count method in Eucalyptus and Juniperus forest, and line transect technique in grassland and moorland habitats were used to study avian diversity. Data were collected in the early morning (6:30 to 9:30 a.m.) and late afternoon (4:30 to 7:00 p.m.) when the activities of birds were prominent. Species diversity and evenness was given in terms of Shannon-Weaver diversity Index. A total of 86 avian species belonging to 14 orders and 35 families were identified. The identified areas are rich with seven (8.14%) endemic bird species namely; abyssinian catbird (Parophasma galinieri), abyssinian longclaw (Macronyx flavicollis), ankober serin (Crithagra ankoberensis), black-headed siskin (Serinus nigriceps), blue-winged goose (Cyanochen cyanoptera), moorland francolin (Scleroptlia psilolaema), spot-breasted plover (Vanellus melanocephalus), and five (5.81%) near-endemic bird species including rouget's rail (Rougetius rougetii), wattled ibis (Bostrychia carunculata), white-collared pigeon (Columba albitorques), thick-billed raven (Corvus crassirostris), and white-winged cliff chat (Myrmecocichla semirufa). -

Bubulcus Ibis (Cattle Egret) European Red List of Birds Supplementary Material
Bubulcus ibis (Cattle Egret) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 3 Sources of reported national population data p. 5 Species factsheet bibliography p. 7 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Bubulcus ibis (Cattle Egret) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 Long-term population trend4 Subspecific population (where relevant) 2 territory) Size (pairs)3 Europe (%) Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Armenia 30-80 <1 2002-2012 good ? ? Azerbaijan 3,500-7,000 6 2014 poor - 2000-2014 - 1980-2014 poor Belgium 0-1 <1 2008-2012 medium 0 0 2000-2012 good ? B. -

2008 - 2015 Wildlife Strike Analyses (Ibis)
International Civil Aviation Organization ELECTRONIC BULLETIN For information only EB 2017/25 12 May 2017 2008 - 2015 WILDLIFE STRIKE ANALYSES (IBIS) The analyses of wildlife strike reports for the years 2008 to 2015 are based on 97 751 reports, received from ninety-one States on strikes occurring in 105 States and territories as shown at Attachment A. A summary of wildlife strikes reported to the ICAO Bird Strike Information System (IBIS) for the years 2008 to 2015 is included at Attachment B, IBIS World Wildlife Strike Statistics at Attachment C and a list of wildlife types at Attachment D. The above attachments (available in English only) can be found at www.icao.int/IBIS. The analyses of wildlife strike data and observing and monitoring of wildlife activities can reveal trends that will assist airport authorities in identifying areas of concern, which should be addressed through a well-managed wildlife control programme. Wildlife strike statistics can also be analysed to determine those times of year or day when wildlife control is needed the most. In order to better facilitate occurrence reporting and data analysis, ICAO now has replaced the old IBIS computer-application with a new reporting system based on the European Co-ordination Centre for Accident and Incident Reporting Systems (ECCAIRS) platform. A User Manual and Software Installation Manual can be downloaded at www.icao.int/IBIS. States are encouraged to submit wildlife strike reports either via ECCAIRS e5f/e4f files, or via an ECCAIRS Excel-based form that can also be downloaded at www.icao.int/IBIS. Enclosures: A — List of States and Territories for the years 2008 - 2015 B — Summary of Wildlife Strikes reported to ICAO Bird Strike Information System (IBIS) for the years 2008 - 2015 C — IBIS World Wildlife Strike Statistics 2008 - 2015 D — List of wildlife types for the years 2008 - 2015 Issued under the authority of the Secretary General 999 Robert-Bourassa Boulevard Tel.: +1 514-954-8219 ext. -

16-DAY SUBTROPICAL SOUTH AFRICA TRIP REPORT, 10 – 25 March 2017
SOUTH AFRICA: 16‐DAY SUBTROPICAL SOUTH AFRICA TRIP REPORT, 10 – 25 March 2017 By Jason Boyce Drakensberg Rockjumper – One of the birds of the trip! www.birdingecotours.com [email protected] 2 | T R I P R E P O R T Subtropical South Africa Trip Report March 2017 TOUR ITINERARY Overnight Day 1 – Arrival and birding Umhlanga Gateway Country Lodge, Umhlanga Day 2 – Umhlanga to Underberg KarMichael Guest Farm, Himeville Day 3 – Sani Pass KarMichael Guest Farm, Himeville Day 4 – Southern Drakensberg to Eshowe Birds of Paradise B&B, Eshowe Day 5 – Ongoye, Mtunzini and Amatikulu Birds of Paradise B&B, Eshowe Day 6 – Eshowe, Dlinza to St Lucia Ndiza Lodge, St Lucia Day 7 – St Lucia Wetland Park Ndiza Lodge, St Lucia Day 8 – St Lucia to Mkhuze Game Reserve Mantuma Camp, Mkhuze Day 9 – Mkhuze Game Reserve Mantuma Camp, Mkhuze Day 10 – Mkhuze to Wakkerstroom Wetlands Country House, Wakkerstroom Day 11 – Wakkerstroom birding Wetlands Country House, Wakkerstroom Day 12 – Wakkerstroom to Skukuza, KNP Kruger National Park, Skukuza Day 13 – Southern Kruger National Park Kruger National Park, Skukuza Day 14 – Kruger National Park to Dullstroom Linger Longer, Dullstroom Day 15 – Dullstroom to Dinokeng Game Reserve Leopardsong Game Lodge, Dinokeng Day 16 – Rust de Winter to Johannesburg airport Flight home OVERVIEW This was a tour with incredible diversity, varying habitats, enjoyable company, and a host of endemic South African bird species. Our 16-day ‘Subtropical South Africa’ tour gave us 397 species of birds, with an additional 15 species being heard only. We also saw 37 mammal species, interesting reptiles, and a few rare South African butterflies. -

Bird Species Diversity and Distribution in Case of Protected Area
ANALYSIS Vol. 20, 2019 ANALYSIS ARTICLE ISSN 2319–5746 EISSN 2319–5754 Species Bird species diversity and distribution in case of protected area Kassahun Abie1, Belete Tilahun1, Abel Feyisa1, Tewodros Kumssa2, Alemneh Amare2 1. Department of Wildlife and Ecotourism Management, Wolkite University, P.O. Box: 07, Wolkite, Ethiopia 2. Department of Biology, Kotebe Metropolitan University, P.O. Box: 31248, Addis Ababa, Ethiopia Corresponding author: Department of Wildlife and Ecotourism Management, Wolkite University, P.O. Box: 07, Wolkite, Ethiopia Email: [email protected] Article History Received: 10 February 2019 Accepted: 21 March 2019 Published: April 2019 Citation Kassahun Abie, Belete Tilahun, Abel Feyisa, Tewodros Kumssa, Alemneh Amare. Bird species diversity and distribution in case of protected area. Species, 2019, 20, 90-100 Publication License This work is licensed under a Creative Commons Attribution 4.0 International License. General Note Article is recommended to print as color digital version in recycled paper. ABSTRACT Study on bird species diversity and distribution is important for conservation efforts in different protected area. However, bird diversity and distribution is little known in Ethiopia. The study area was stratified into five habitat types based on land cover features. Point transect count was employed gently up to 30 m radius with random sampling technique. The data were analyzed by one-way ANOVA. This study revealed that a total of 112 species were identified. Of which two endemic to Ethiopia, five near endemic, 69 90 common residences, 22 Palearctic and 12 Intra-Africa migratory bird species were recorded. The mean of bird species abundance Page was significantly different between dry and wet seasons (f= 4.8, p<0.05, df = 1).