C-Jun N-Terminal Kinase Primes Endothelial Cells at Atheroprone Sites for Apoptosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tak1 Polyclonal Antibody Catalog # AP72719
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 Tak1 Polyclonal Antibody Catalog # AP72719 Specification Tak1 Polyclonal Antibody - Product Information Application IF Primary Accession O43318 Reactivity Human, Mouse, Rat Host Rabbit Clonality Polyclonal Tak1 Polyclonal Antibody - Additional Information Gene ID 6885 Other Names MAP3K7; TAK1; Mitogen-activated protein kinase kinase kinase 7; Transforming growth factor-beta-activated kinase 1; TGF-beta-activated kinase 1 Dilution IF~~IF: 1:50-200 Western Blot: 1/500 - 1/2000. Immunohistochemistry: 1/100 - 1/300. ELISA: 1/40000. Not yet tested in other applications. Format Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide. Storage Conditions -20℃ Tak1 Polyclonal Antibody - Protein Information Name MAP3K7 {ECO:0000303|PubMed:28397838, ECO:0000312|HGNC:HGNC:6859} Function Serine/threonine kinase which acts as an essential component of the MAP kinase signal transduction pathway. Plays an important role in the cascades of cellular responses evoked by changes in the environment. Mediates signal transduction of TRAF6, various cytokines including Page 1/3 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 interleukin-1 (IL-1), transforming growth factor-beta (TGFB), TGFB-related factors like BMP2 and BMP4, toll-like receptors (TLR), tumor necrosis factor receptor CD40 and B-cell receptor (BCR). Ceramides are also able to activate MAP3K7/TAK1. Once activated, acts as an upstream activator of the MKK/JNK signal transduction cascade and the p38 MAPK signal transduction cascade through the phosphorylation and activation of several MAP kinase kinases like MAP2K1/MEK1, MAP2K3/MKK3, MAP2K6/MKK6 and MAP2K7/MKK7. -

Wo 2010/075007 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075007 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12Q 1/68 (2006.01) G06F 19/00 (2006.01) kind of national protection available): AE, AG, AL, AM, C12N 15/12 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/067757 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 11 December 2009 ( 11.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 12/3 16,877 16 December 2008 (16.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): DODDS, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, W., Jean [US/US]; 938 Stanford Street, Santa Monica, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, CA 90403 (US). -

Genome-Wide Analysis of Host-Chromosome Binding Sites For
Lu et al. Virology Journal 2010, 7:262 http://www.virologyj.com/content/7/1/262 RESEARCH Open Access Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) Fang Lu1, Priyankara Wikramasinghe1, Julie Norseen1,2, Kevin Tsai1, Pu Wang1, Louise Showe1, Ramana V Davuluri1, Paul M Lieberman1* Abstract The Epstein-Barr Virus (EBV) Nuclear Antigen 1 (EBNA1) protein is required for the establishment of EBV latent infection in proliferating B-lymphocytes. EBNA1 is a multifunctional DNA-binding protein that stimulates DNA replication at the viral origin of plasmid replication (OriP), regulates transcription of viral and cellular genes, and tethers the viral episome to the cellular chromosome. EBNA1 also provides a survival function to B-lymphocytes, potentially through its ability to alter cellular gene expression. To better understand these various functions of EBNA1, we performed a genome-wide analysis of the viral and cellular DNA sites associated with EBNA1 protein in a latently infected Burkitt lymphoma B-cell line. Chromatin-immunoprecipitation (ChIP) combined with massively parallel deep-sequencing (ChIP-Seq) was used to identify cellular sites bound by EBNA1. Sites identified by ChIP- Seq were validated by conventional real-time PCR, and ChIP-Seq provided quantitative, high-resolution detection of the known EBNA1 binding sites on the EBV genome at OriP and Qp. We identified at least one cluster of unusually high-affinity EBNA1 binding sites on chromosome 11, between the divergent FAM55 D and FAM55B genes. A con- sensus for all cellular EBNA1 binding sites is distinct from those derived from the known viral binding sites, sug- gesting that some of these sites are indirectly bound by EBNA1. -

Characterization of Genome-Wide TFCP2 Targets In
Xu et al. Journal of Experimental & Clinical Cancer Research (2015) 34:6 DOI 10.1186/s13046-015-0121-1 RESEARCH Open Access Characterization of genome-wide TFCP2 targets in hepatocellular carcinoma: implication of targets FN1 and TJP1 in metastasis Xiao Xu1†, Zhikun Liu1†, Lin Zhou2, Haiyang Xie2, Jun Cheng1, Qi Ling1, Jianguo Wang2, Haijun Guo1, Xuyong Wei2 and Shusen Zheng1* Abstract Background: Transcription factor CP2 (TFCP2) is overexpressed in hepatocellular carcinoma(HCC) and correlated with the progression of the disease. Here we report the use of an integrated systems biology approach to identify genome-wide scale map of TFCP2 targets as well as the molecular function and pathways regulated by TFCP2 in HCC. Methods: We combined Chromatin immunoprecipitation (ChIP) on chip along with gene expression microarrays to study global transcriptional regulation of TFCP2 in HCC. The biological functions, molecular pathways, and networks associated with TFCP2 were identified using computational approaches. Validation of selected target gene expression and direct binding of TFCP2 to promoters were performed by ChIP -PCR and promoter reporter. Results: TFCP2 fostered a highly aggressive and metastatic phenotype in different HCC cells. Transcriptome analysis showed that alteration of TFCP2 in HCC cells led to change of genes in biological functions involved in cancer, cellular growth and proliferation, angiogenesis, cell movement and attachment. Pathways related to cell movement and cancer progression were also enriched. A quest for TFCP2-regulated factors contributing to metastasis, by integration of transcriptome and ChIP on chip assay, identified fibronectin 1 (FN1) and tight junction protein 1 (TJP1) as targets of TFCP2, and as key mediators of HCC metastasis. -

Protein Phosphorylation Sites Notes References Transcription Factors
Protein Phosphorylation Notes References sites Transcription AML1 Ser249, Ser266 Phosphorylation of the acute myelogenous leukemia 1 (AML1) potentially regulates its (Tanaka et al., 1996) Factors (RUNX1) transactivation potential and thereby modifies myeloid hematopoietic differentiation. Androgen Ser514 Phosphorylation of androgen receptor by ERKs is controversial. May play a role in survival (Yeh et al., 1999) receptor and proliferation of prostate cells. Arix/Phox2a Ser36, Ser71 Phosphorylation of Arix by ERK1/2 inhibits its ability to interact with target genes. (Hsieh et al., 2005) ATF2 Thr71 This phosphorylation together with the phosphorylation of Thr69 by p38MAPK induces (Ouwens et al., 2002) activation of ATF2. Beta2 (Neuro Ser274 Phosphorylation of this HLH transcription factor induces its glucose-sensitive transactivation (Khoo et al. 2003) D1) and enhances its capability to heterodimerize with E47/12 and bind to DNA. BCL-6 Ser333, Ser343 Phosphorylation of BCL-6 induces its degradation that is important for B-cell differentiation (Niu et al., 1998) and antibody response. BMAL1 Ser527, Thr534, Phosphorylation of this helix-loop-helix-PAS transcription factor inhibits its activity as a clock (Sanada et al., 2002) Ser599 (chicken) oscillator. CBP Thr188 Phosphorylation of the C-terminal region of the CREB binding protein (CBP, and also its (Janknecht and homolog p300) enhances its transactivation potential. Nordheim, 1996) C/EBPb Thr188 (rat) Phosphorylation of the CCAAT/enhancer-binding protein-b (C/EBPb) by ERK2 (not ERK1) (Hanlon et al., 2001) enhances its interaction with SRF and its transactivation activity. CRY1/2 Ser265, Ser557 Phosphorylation of the Cryptochromes proteins CRY1 and 2 results in attenuation of their (Sanada et al., 2004) (CRY1, mouse) ability to inhibit BMAL1:CLOCK-mediated transcription. -

2219.Full.Pdf
The JNK Are Important for Development and Survival of Macrophages S. Roy Himes, David P. Sester, Timothy Ravasi, Stephen L. Cronau, Tedjo Sasmono and David A. Hume This information is current as of September 28, 2021. J Immunol 2006; 176:2219-2228; ; doi: 10.4049/jimmunol.176.4.2219 http://www.jimmunol.org/content/176/4/2219 Downloaded from References This article cites 64 articles, 40 of which you can access for free at: http://www.jimmunol.org/content/176/4/2219.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 28, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2006 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology The JNK Are Important for Development and Survival of Macrophages S. Roy Himes, David P. Sester, Timothy Ravasi, Stephen L. Cronau, Tedjo Sasmono, and David A. Hume1 We report in this study that activation of the JNK by the growth factor, CSF-1 is critical for macrophage development, prolif- eration, and survival. -

O-Glcnacylated C-Jun Antagonizes Ferroptosis Via Inhibiting GSH Synthesis in Liver Cancer T
Cellular Signalling 63 (2019) 109384 Contents lists available at ScienceDirect Cellular Signalling journal homepage: www.elsevier.com/locate/cellsig O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer T Yan Chena, Guoqing Zhua, Ya Liua,QiWua, Xiao Zhangb, Zhixuan Bianc, Yue Zhangd, ⁎ ⁎ Qiuhui Panc, , Fenyong Suna, a Department of Clinical Laboratory Medicine, Shanghai Tenth People's Hospital of Tongji University, Shanghai 200072, China b Shanghai Institute of Thoracic Tumors, Shanghai Chest Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200030, China c Department of Laboratory Medicine, Shanghai Children's Medical Center, Shanghai Jiaotong University School of Medicine, Shanghai 200127, China d Department of Central Laboratory, Shanghai Tenth People's Hospital of Tongji University, Shanghai 200072, China ARTICLE INFO ABSTRACT Keywords: Ferroptosis is a metabolism-related cell death. Stimulating ferroptosis in liver cancer cells is a strategy to treat Erastin liver cancer. However, how to eradicate liver cancer cells through ferroptosis and the obstacles to inducing O-GlcNAcylation ferroptosis in liver cancer remain unclear. Here, we observed that erastin suppressed the malignant phenotypes Phosphorylation of liver cancer cells by inhibiting O-GlcNAcylation of c-Jun and further inhibited protein expression, tran- Glutathione scription activity and nuclear accumulation of c-Jun. Overexpression of c-Jun-WT with simultaneous PuGNAc Transcription treatment conversely inhibited erastin-induced ferroptosis, whereas overexpression of c-Jun-WT alone or Promoter overexpression of c-Jun-S73A (a non-O-GlcNAcylated form of c-Jun) with PuGNAc treatment did not exert a similar effect. GSH downregulation induced by erastin was restored by overexpression of c-Jun-WT with si- multaneous PuGNAc treatment. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Genomic Analyses of PMBL Reveal New Drivers and Mechanisms of Sensitivity to PD-1 Blockade
Regular Article LYMPHOID NEOPLASIA Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade Bjoern Chapuy,1,2,* Chip Stewart,3,* Andrew J. Dunford,3,* Jaegil Kim,3 Kirsty Wienand,1 Atanas Kamburov,3 Gabriel K. Griffin,4 Pei-Hsuan Chen,4 Ana Lako,4 Robert A. Redd,5 Claire M. Cote,1 Matthew D. Ducar,6 Aaron R. Thorner,6 Scott J. Rodig,4 Gad Getz,3,7,8,† Downloaded from https://ashpublications.org/blood/article-pdf/134/26/2369/1549702/bloodbld2019002067.pdf by Margaret Shipp on 30 December 2019 and Margaret A. Shipp1,† 1Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA; 2Department of Hematology and Oncology, University Medical Center Gottingen,¨ Gottingen,¨ Germany; 3Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, MA; 4Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 5Department of Data Sciences and 6Center for Cancer Genome Discovery, Dana-Farber Cancer Institute, Boston, MA; and 7Department of Pathology and 8Center for Cancer Research, Massachusetts General Hospital, Harvard Medical School, Boston, MA KEY POINTS Primary mediastinal large B-cell lymphomas (PMBLs) are aggressive tumors that typically present as large mediastinal masses in young women. PMBLs share clinical, transcriptional, l Comprehensive genomic analyses of and molecular features with classical Hodgkin lymphoma (cHL), including constitutive PMBL reveal new activation of nuclear factor kB (NF-kB), JAK/STAT signaling, and programmed cell death genetic drivers such protein 1 (PD-1)–mediated immune evasion. The demonstrated efficacy of PD-1 blockade in as ZNF217. relapsed/refractory PMBLs led to recent approval by the US Food and Drug Administration l High mutational and underscored the importance of characterizing targetable genetic vulnerabilities in this burden, MSI, and disease. -

Transcriptome Analysis of Human Diabetic Kidney Disease
ORIGINAL ARTICLE Transcriptome Analysis of Human Diabetic Kidney Disease Karolina I. Woroniecka,1 Ae Seo Deok Park,1 Davoud Mohtat,2 David B. Thomas,3 James M. Pullman,4 and Katalin Susztak1,5 OBJECTIVE—Diabetic kidney disease (DKD) is the single cases, mild and then moderate mesangial expansion can be leading cause of kidney failure in the U.S., for which a cure has observed. In general, diabetic kidney disease (DKD) is not yet been found. The aim of our study was to provide an considered a nonimmune-mediated degenerative disease unbiased catalog of gene-expression changes in human diabetic of the glomerulus; however, it has long been noted that kidney biopsy samples. complement and immunoglobulins sometimes can be de- — tected in diseased glomeruli, although their role and sig- RESEARCH DESIGN AND METHODS Affymetrix expression fi arrays were used to identify differentially regulated transcripts in ni cance is not clear (4). 44 microdissected human kidney samples. The DKD samples were The understanding of DKD has been challenged by multi- significant for their racial diversity and decreased glomerular ple issues. First, the diagnosis of DKD usually is made using filtration rate (~20–30 mL/min). Stringent statistical analysis, using clinical criteria, and kidney biopsy often is not performed. the Benjamini-Hochberg corrected two-tailed t test, was used to According to current clinical practice, the development of identify differentially expressed transcripts in control and diseased albuminuria in patients with diabetes is sufficient to make the glomeruli and tubuli. Two different Web-based algorithms were fi diagnosis of DKD (5). We do not understand the correlation used to de ne differentially regulated pathways. -
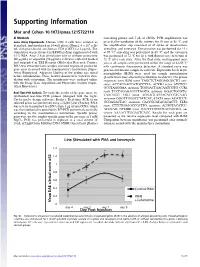
Supporting Information
Supporting Information Mor and Cohen 10.1073/pnas.1215722110 SI Methods containing primer and 5 μL of cDNA. PCR amplification was Gene Array Experiments. Human CD4 T cells were isolated as preceded by incubation of the mixture for 10 min at 95 °C, and described, and incubated in 24-well plates (Nunc), 4 × 106 cells/ the amplification step consisted of 45 cycles of denaturation, mL with plate-bound anti-human CD3 (OKT3) at 2 μg/mL. The annealing, and extension. Denaturation was performed for 15 s stimulation was performed in RPMI medium supplemented with at 95 °C; annealing was performed in 60 °C; and the extension 0.1% BSA. After 2 h of stimulation with or without cefuroxime was performed at 72 °C for 20 s, with fluorescence detection at (50 μg/mL) or ampicillin (50 μg/mL), cells were collected washed 72 °C after each cycle. After the final cycle, melting-point anal- and suspended in TRI Reagent (Molecular Research Center). yses of all samples were performed within the range of 62–95 °C RNA was extracted from samples and used to prepare probes for with continuous fluorescence detection. A standard curve was gene array in accord with the manufacturer’s instructions (Super- generated from one sample in each run. Expression levels of β2- Array Bioscience). Adequate labeling of the probes was tested microglobulin (B2M) were used for sample normalization before hybridization. Three healthy donors were tested in stim- (β-actin levels were affected by cefuroxime treatment). The primer ulation with cefuroxime. The membranes were analyzed online sequences were B2M sense TAGCTCTAGGAGGGCTG anti- with the Image Data Acquisition and Expression Analysis (Super- sense ACCACAACCATGCCTTA; ACVR2 sense ATCTCC- Array Bioscience). -

Genome-Wide Identification of Genes Regulating DNA Methylation Using Genetic Anchors for Causal Inference
bioRxiv preprint doi: https://doi.org/10.1101/823807; this version posted October 30, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Genome-wide identification of genes regulating DNA methylation using genetic anchors for causal inference Paul J. Hop1,2, René Luijk1, Lucia Daxinger3, Maarten van Iterson1, Koen F. Dekkers1, Rick Jansen4, BIOS Consortium5, Joyce B.J. van Meurs6, Peter A.C. ’t Hoen 7, M. Arfan Ikram8, Marleen M.J. van Greevenbroek9,10, Dorret I. Boomsma11, P. Eline Slagboom1, Jan H. Veldink2, Erik W. van Zwet12, Bastiaan T. Heijmans1* 1 Molecular Epidemiology, Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, 2333 ZC, The Netherlands 2 Department of Neurology, UMC Utrecht Brain Center, University Medical Centre Utrecht, Utrecht University, Utrecht 3584 CG, Netherlands 3 Department of Human Genetics, Leiden University Medical Center, Leiden, 2333 ZC, The Netherlands 4 Department of Psychiatry, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam Neuroscience, Amsterdam, 1081 HV, The Netherlands 5 Biobank-based Integrated Omics Study Consortium. For a complete list of authors, see the acknowledgements. 6 Department of Internal Medicine, Erasmus Medical Centre, Rotterdam, The Netherlands. 7 Centre for Molecular and Biomolecular Informatics, Radboud Institute for Molecular Life Sciences, Radboud University