Temporary Policy for Preparation of Alcohol-Based Hand Sanitizer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hand Hygiene Solutions
PRODUCT OVERVIEWS Hand Hygiene Solutions Healing Hands Need Help, Too. Almost 80% of infectious Hand hygiene compliance Product selection matters diseases are transmitted rates are often very low Products containing aloe vera, via touch Outpatient clinics: Studies vitamin E and other emollients One of the most common have shown that hand hygiene can help to soothe and protect modes of transmission is frequency amongst clinic staff hands and even promote hand via the hands of healthcare ranges from 11-50%.2, 3 hygiene compliance.4 professionals.1 Designed for the demanding needs of healthcare professionals, Clorox Healthcare® provides a wide array of hand hygiene solutions formulated for frequent hand hygiene in healthcare settings. References 1. Tierno, P. The Secret Life of Germs. New York, NY, USA: Atria Books, 2001. 2. Kukanich KS, Kaur R, Freeman LC, Powell DA. Evaluation of a hand hygiene campaign in outpatient health care clinics. Am J Nurs. 2013 Mar;113(3):36-42. 3. Thompson D, Bowdey L, Brett M, Cheek, Using medical student observers of infection prevention, hand hygiene, and injection safety in outpatient settings: A cross-sectional survey. J Am J Infect. Control 2016; 44(4):374–380. 4. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. 2009. https://www.ncbi.nlm.nih.gov/books/NBK144051/ (Accessed 22 February 2017) 5. CDC. “Handwashing: Clean Hands Save Lives-Show Me the Science-Why Wash Your Hands?” https://www.cdc.gov/handwashing/why-handwashing.html (Accessed 15 March 2017). Hand hygiene is one of the most effective ways to stop the spread of germs and keep patients, staff and visitors safe.5 Our portfolio of hand hygiene solutions can be used to clean and soothe hands throughout your facility-from reception areas, to patient care areas to break rooms and offices. -

Ethanol Rins and Hand Sanitizer
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY NATIONAL VEHICLE AND FUEL EMISSIONS LABORATORY 2565 PLYMOUTH ROAD ANN ARBOR, MICHIGAN 48105-2498 OFFICE OF AIR AND RADIATION May 11, 2020 Re: Ethanol RINs and Hand Sanitizer Production for EP3 Pathway Ethanol Facilities during the COVID-19 Public Health Emergency Dear Ethanol Producers: EPA administers the Renewable Fuel Standard (RFS) program, including determining what volumes of fuel ethanol qualify for tradable credits (RINs) under the program. In 2014, as part of the RFS Program in 40 C.F.R. Part 80, we created the Efficient Producer Petition Process (EP3) as a streamlined process for corn starch and grain sorghum ethanol producers to demonstrate that their fuel satisfies the lifecycle greenhouse gas (GHG) reduction requirements to qualify for RINs. Consistent with 40 C.F.R. § 80.1416, over 80 dry mill ethanol plants in the United States have been approved for EP3 pathways. Given the current 2019 coronavirus disease (COVID-19), we understand that some consumers and health care professionals are currently experiencing difficulties accessing alcohol-based hand sanitizers. To enhance the availability of hand sanitizer products, the Food and Drug Administration (FDA) has issued a number of temporary policies, including temporary guidance for producers of alcohol for hand sanitizer.1 Based on input from industry, we understand fuel ethanol facilities can quickly and significantly enhance the availability of alcohol suitable for hand sanitizer production. Ethanol production through an EP3 pathway is limited to 200-proof ethanol, whereas alcohol suitable for use as an ingredient in hand sanitizer (hand sanitizer alcohol) is commonly produced as 190-proof ethanol. -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Hand Hygiene: Clean Hands for Healthcare Personnel
Core Concepts for Hand Hygiene: Clean Hands for Healthcare Personnel 1 Presenter Russ Olmsted, MPH, CIC Director, Infection Prevention & Control Trinity Health, Livonia, MI Contributions by Heather M. Gilmartin, NP, PhD, CIC Denver VA Medical Center University of Colorado Laraine Washer, MD University of Michigan Health System 2 Learning Objectives • Outline the importance of effective hand hygiene for protection of healthcare personnel and patients • Describe proper hand hygiene techniques, including when various techniques should be used 3 Why is Hand Hygiene Important? • The microbes that cause healthcare-associated infections (HAIs) can be transmitted on the hands of healthcare personnel • Hand hygiene is one of the MOST important ways to prevent the spread of infection 1 out of every 25 patients has • Too often healthcare personnel do a healthcare-associated not clean their hands infection – In fact, missed opportunities for hand hygiene can be as high as 50% (Chassin MR, Jt Comm J Qual Patient Saf, 2015; Yanke E, Am J Infect Control, 2015; Magill SS, N Engl J Med, 2014) 4 Environmental Surfaces Can Look Clean but… • Bacteria can survive for days on patient care equipment and other surfaces like bed rails, IV pumps, etc. • It is important to use hand hygiene after touching these surfaces and at exit, even if you only touched environmental surfaces Boyce JM, Am J Infect Control, 2002; WHO Guidelines on Hand Hygiene in Health Care, WHO, 2009 5 Hands Make Multidrug-Resistant Organisms (MDROs) and Other Microbes Mobile (Image from CDC, Vital Signs: MMWR, 2016) 6 When Should You Clean Your Hands? 1. Before touching a patient 2. -

Bio-Butanol Production from Glycerol with Clostridium
Lin et al. Biotechnol Biofuels (2015) 8:168 DOI 10.1186/s13068-015-0352-6 RESEARCH Open Access Bio‑butanol production from glycerol with Clostridium pasteurianum CH4: the effects of butyrate addition and in situ butanol removal via membrane distillation De‑Shun Lin1, Hong‑Wei Yen2, Wei‑Chen Kao1, Chieh‑Lun Cheng1, Wen‑Ming Chen3, Chieh‑Chen Huang4 and Jo‑Shu Chang1,4,5* Abstract Background: Clostridium pasteurianum CH4 was used to produce butanol from glycerol. The performance of butanol fermentation was improved by adding butyrate as the precursor to trigger the metabolic pathway toward butanol production, and by combining this with in situ butanol removal via vacuum membrane distillation (VMD) to avoid the product inhibition arising from a high butanol concentration. 1 Results: Adding 6 g L− butyrate as precursor led to an increase in the butanol yield from 0.24 to 0.34 mol butanol 1 (mol glycerol)− . Combining VMD and butyrate addition strategies could further enhance the maximum effective 1 1 butanol concentration to 29.8 g L− , while the yield was also improved to 0.39 mol butanol (mol glycerol)− . The butanol concentration in the permeate of VMD was nearly five times higher than that in the feeding solution. Conclusions: The proposed butyrate addition and VMD in situ butanol removal strategies are very effective in enhancing both butanol titer and butanol yield. This would significantly enhance the economic feasibility of fermen‑ tative production of butanol. The VMD-based technology not only alleviates the inhibitory effect of butanol, but also markedly increases butanol concentration in the permeate after condensation, thereby making downstream process‑ ing easier and more cost-effective. -

Presentation on Key Ideas of Elementary Mathematics
Key Ideas of Elementary Mathematics Sybilla Beckmann Department of Mathematics University of Georgia Lesson Study Conference, May 2007 Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 1/52 US curricula are unfocused A Splintered Vision, 1997 report based on the TIMSS curriculum analysis. US state math curriculum documents: “The planned coverage included so many topics that we cannot find a single, or even a few, major topics at any grade that are the focus of these curricular intentions. These official documents, individually or as a composite, are unfocused. They express policies, goals, and intended content coverage in mathematics and the sciences with little emphasis on particular, strategic topics.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 2/52 US instruction is unfocused From A Splintered Vision: “US eighth grade mathematics and science teachers typically teach far more topic areas than their counterparts in Germany and Japan.” “The five surveyed topic areas covered most extensively by US eighth grade mathematics teachers accounted for less than half of their year’s instructional periods. In contrast, the five most extensively covered Japanese eighth grade topic areas accounted for almost 75 percent of their year’s instructional periods.” Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 3/52 Breaking the “mile-wide-inch-deep” habit Every mathematical skill and concept has some useful application has some connection to other concepts and skills So what mathematics should we focus on? Sybilla Beckmann (University of Georgia) Key Ideas of Elementary Mathematics 4/52 What focus? Statistics and probability are increasingly important in science and in the modern workplace. -

Chemistry and New Uses of Sucrose: How Important?
Pure & Appi. Chem., Vol. 56, No. 7, pp. 833—844, 1984. 0033—4545/84 $3.OO+O.OO Printed in Great Britain. Pergamon Press Ltd. ©1984 IUPAC CHEMISTRY AND NEW USES OF SUCROSE: HOW IMPORTANT? Riaz Khan Philip Lyle Memorial Research Laboratory, Tate & Lyle PLC, Whiteknights P.O. Box 68, Reading, England Abstract —Somerecent work on selective reactions of sucrose is described. These reactions reveal a profile of chemical reactivity which has been exploited to produce a number of products of commercial significance. The potential of sucrose as a raw material for chemicals and energy is also indicated. INTRODUCTION The world economy is critically dependent upon oil which is a non—regenerable material. In order to maintain an economic order in the world it is therefore imperative that the future energy and chemical needs are secured. In the short—term we must curb on wasteful consumption of oil and in the long—term dependence on oil must be reduced. Alternative resources such as coal, tarsands, shale oil, natural gas and lignite, agricultural and aquatic materials must be considered as sources of energy and chemicals. The potential of carbohydrates as feedstocks for chemicals has been demonstrated, but not yet significantly commercially realised except in a few instances. Although cellulose is the most abundant carbohydrate, several technical difficulties in its processing makes it economically unattractive as a raw material for energy and chemicals. In contrast sucrose and starch are readily amenable to chemical and biochemical modifications giving a range of compounds presently derived through petrochemical routes, as well as new derivatives of potential commercial significance. -
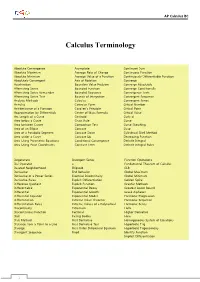
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Natured Alcohol and Spe- Cially Denatured
Alcohol and Tobacco Tax and Trade Bureau, Treasury § 21.141 (c) Purity. Technical grade or better. droxide. Reflux for 1 hour on a steam bath, cool and titrate the excess so- [T.D. ATF–133, 48 FR 24673, June 2, 1983. Re- designated by T.D. ATF–442, 66 FR 12854, dium hydroxide with 0.5 N sulfuric acid Mar. 1, 2001] using phenolphthalein indicator. Percent sucrose octaacetate=(ml NaOH¥ml § 21.129 Spearmint oil, terpeneless. H2SO4)×4.2412/weight of sample (a) Carvone content. Not less than 85 percent by weight. [T.D. ATF–133, 48 FR 24673, June 2, 1983. Re- ° designated by T.D. ATF–442, 66 FR 12854, (b) Refractive index at 20 C. 1.4930 to Mar. 1, 2001] 1.4980. (c) Specific gravity at 25 °/25 °C. 0.949 to § 21.132 Toluene. 0.956. (d) Odor. Characteristic odor. (a) Distillation range. (For applicable ASTM method, see 1980 Annual Book of [T.D. ATF–133, 48 FR 24673, June 2, 1983. Re- ASTM Standards, Part 29, page 569, designated by T.D. ATF–442, 66 FR 12854, Standard No. D 362–75 for industrial Mar. 1, 2001] grade toluene; for incorporation by ref- § 21.130 Spike lavender oil, natural. erence, see § 21.6(b).) When 100 ml of tol- uene are distilled by this method, not (a) Alcohol content (as borneol). Not more than 1 ml should distill below 109 less than 30 percent by weight. °C., and not less than 99 ml below 112 (b) Esters (as bornyl acetate). Not less °C. than 1.5 percent by weight. -

FDA Warns That Vapors from Alcohol-Based Hand Sanitizers Can Have Side Effects Apply Hand Sanitizer in a Well-Ventilated Area
FDA warns that vapors from alcohol-based hand sanitizers can have side effects Apply hand sanitizer in a well-ventilated area 6-16-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that symptoms such as headache, nausea, and dizziness can occur after applying alcohol-based hand sanitizers to the skin. These symptoms are likely to have occurred because of vapors from the hand sanitizer, potentially from exposure in enclosed spaces or places with poor air circulation. We have received increasing reports of these side effects since the start of the COVID-19 pandemic. Most people experienced minor or minimal effects; however, some cases required treatment by a health care professional. What is FDA doing? We are continuing to monitor reports of adverse events that occur with hand sanitizers. At this time, we are not making any changes to the Drug Facts label for hand sanitizers but will inform the public if additional information becomes available. What are hand sanitizers and how can they help me? Hand sanitizers are over-the-counter (OTC) drug products that can help consumers reduce bacteria on their hands. However, the best way to prevent the spread of infections and decrease the risk of getting sick is by washing your hands with plain soap and water, advises the Centers for Disease Control and Prevention (CDC). Washing hands often with soap and water for at least 20 seconds is essential, especially after going to the restroom; before eating; and after coughing, sneezing, or blowing one’s nose. -

SDS Contains All of the Information Required by the HPR
SAFETY DATA SHEET Preparation Date: 01/26/2015 Revision Date: 7/25/2018 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: SU104 Product Name: SUCROSE OCTAACETATE, NF Other means of identification Synonyms: alpha-d-glucopyranoside; 1,3,4,6-tetra-O-acetyl-beta-D-Frutofuranosyl; tetraacetate; Octaacetylsucrose CAS #: C28H38O19 RTECS # WN6620000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: No information available. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is not considered hazardous according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Not a dangerous substance or mixture according to the Globally Harmonized System (GHS) Label elements Not classified Hazards not otherwise classified (HNOC) Not Applicable Other hazards Not available Product code: SU104 Product name: SUCROSE 1 / 10 OCTAACETATE, NF 3. COMPOSITION/INFORMATION ON INGREDIENTS Components CAS-No. Weight % Sucrose Octaacetate 126-14-7 100 4. FIRST AID MEASURES First aid measures General Advice: National Capital Poison Center in the United States can provide assistance if you have a poison emergency and need to talk to a poison specialist. Call 1-800-222-1222. Skin Contact: Wash off immediately with soap and plenty of water removing all contaminated clothing and shoes. Get medical attention if irritation develops. Consult a physician if necessary. -

A Cooked Composition of Glycerine and Hydrogenated Starch Hydrolysate and the Use Thereof As a Stabilizer for L-Aspartic Acid Sweetening Agent in Comestibles
Europaisches Patentamt 0 323 442 J> European Patent Office Publication number: A1 Office europeen des brevets EUROPEAN PATENT APPLICATION @ Application number: 89102633.8 IntCI* A 23 L 1/236 A 23 G 3/30 @ Date of filing: 27.03.86 2g) Priority: 29.03.85 US 717630 © Applicant: NABISCO BRANDS, INC. 100 DeForest Avenue East Hanover New Jersey 07936 (US) §) Date of publication of application: 05.07.89 Bulletin 89/27 @ Inventor: Carroll, Thomas J. States: BE DE FR GB IT 20-30 43rd Street g) Designated Contracting Astoria, N.Y. (US) jig) Publication number of the earlier application in Kehoe, Gary S. accordance with Art. 76 EPC: 0 196 640 10 Cross Hill Road Ridgefield, Conn. (US) @) Representative : Brauns, Hans-Adolf, Dr. rer. nat. et al Hoffmann, Eitle & Partner, Patentanwalte Arabellastrasse 4 D-8000 Munich 81 (DE) @ A cooked composition of glycerine and hydrogenated starch hydrolysate and the use thereof as a stabilizer for L-aspartic acid sweetening agent in comestibles. (g) A cooked composition of glycerine and hydrogenated starch hydrolysate having a moisture content of 10 ± 6% (W/W). Said composition can be used as a stabilizer for L-aspartic acid sweetening agent in comestibles. CM CO CM eo a. LU Bundesdruckerei Berlin EP 0 323 442 A1 Description A COOKED COMPOSITION OF GLYCERINE AND HYDROGENATED STARCH HYDROLYSATE AND THE USE THEREOF AS A STABILIZER FOR L-ASPARTIC ACID SWEETENING AGENT IN COMESTIBLES Aspartame which is used extensively in many types of sugarless foodstuffs, or other comestible products, 5 such as chewing gum, is known to readily decompose in the presence of moisture into decomposition products such as diketopiperizine which causes a significant loss in the sweetness properties of such products during their shelf lives.