Corbicula Linduensis ERSS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 2, Number 1 (2011), pp. 23-32 © International Research Publication House http://www.irphouse.com A Genetical and Ecological Diversity of Fresh Water Prawns Macrobrachium Canarae and Caridina Gracilirostris from Kanyakumari Dist., Tamil Nadu, India Siva Ranjanee S.1 and Mariapan N.2 1Department of Biotechnology, Vels University, Pallavaram, Chennai, India 2Senior Medical Writer, SIRO Clinpharm Pvt. Ltd., Thane (W) 400 607. India E-mail: [email protected], [email protected] Abstract Fresh water prawns are cultured widely around the world but little is known about the levels and patterns of genetic diversity. This paper reports the RAPD analysis of two species of fresh water prawns of Atydiae and Palaemonidiae family and the genus Macrobrachium and Caridina collected from Kanyakumari district. Specimens are identified using species-specific morphological characteristics. Morphological characters have limitations of describing intra specific genetic diversity as they are polygenic and expressions can be modified by the environment. The advent of molecular technique made possible not only the genetic analysis and also the study of evolutionary relationship. Molecular analysis of the morphologically identified species are done by extracting the DNA from the animal and concentration of DNA is measured using Nanodrop spectrophotometer at a wavelength of 280nm. Further analysis was performed with RAPD (Rapid Amplified Polymorphic DNA) a PCR (Polymerase Chain Reaction) based technique. It consist of genomic DNA, amplified with randomly constructed oligonucleotides. Specific quantity of extracted DNA was then amplified by multiplex PCR using random primers and 16S rRNA gene products was then analyzed using a bioanalyzer. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -

Corbicula Management Plan
Management Plan for Control of Asian Clam (Corbicula fluminea) in the Mukwonago River Watershed Crooked Creek Preserve, Mukwonago River Watershed (Conservancy, 2014). Kara Henderlight, Jennifer Hoelzer, Kara Kehl, Brian McDonald, Erin Peeters, Jason Tutkowski, and Cory Widmayer December 10, 2014 1 Table of Contents Introduction……………………………………………………………………………..…………3 Background…………………………………………………………………………………….….4 Mukwonago River Watershed…………………………………………………………….4 Native Unionid Mussels……………………………………………………….…………..6 Corbicula fluminea………………………………………………………………………..7 Preliminary Assessment…………………………………………………………………………...8 Problem……………………………………………………………………………………...…….9 Goals and Objectives…………………………………………………………………….………11 Management Strategies…………………………………………………………………………..12 Prevention…………………………………………………………………………..……12 Education and Outreach………………………………………………………….………13 Interventions…...……………………………………………………………………...…………14 Lakes: Benthic Barriers and Suction Harvesting……………………………………..….16 Rivers: Manual Removal……………………………………………………………..….18 No Action Plan………………………………………………………………………..….23 Monitoring and Assessment……………………………………………….……………………..24 Resources………………………………………………………………………………...………25 Conclusion…………………………………………………………………………………...…..26 References…………………………………………………………………………….………….28 Figure References………………………………………………………………………….…….33 Appendix A: Grants…………………………………………………………………….………..34 Appendix B: Businesses………………………………………………………………..………..37 Appendix C: Public and Private Grade Schools………………………………………………...39 Appendix D: Environmental Organizations…………………………………………….……….41 -
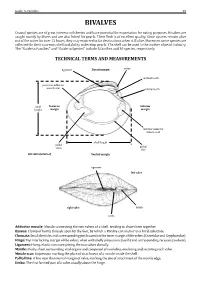
Field Identification Guide to the Living Marine Resources In
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

The Asiatic Clam Corbicula Fluminea (Müller, 1774)
The Asiatic clam Corbicula fluminea (Müller, 1774) (Bivalvia: Corbiculidae) in Europe R. Araujo, D. Moreno and M. A. Ramos Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal, 2. 28006, Madrid, Spain Abstract. Two populations of Corbicula fluminea were found in the Iberian Península; one in Spain and the other in Portugal. A detailed description in terms of ecology shell morphology and microstructure, morphometrics and anatomy is given for the Spanish population from the Mino River. Lectotypes for Tellina fluminea and T. fluminalis, and a neotype for T. fluviatilis are designated and illustrated. Distribuüon and spread of C. fluminea in Europe are revised. Comparisons among some European populations and the populations from Cantón, China, and the Mino River are made. Results suggest that, except for one doubtful population, all records of Corbicula in Europe are attributable to C. fluminea. Corbicula taxonomy begins in 1774 with Müller who Thus, Talavera and Faustino (1933) {In: Britton and described three species in the genus Tellina Linne, 1758: T. Morton, 1979) placed Corbicula manilensis (Philippi, 1844) fluminalis "in fluvio Asiae Euphrat"; T. fluminea "in arena into synonymy with C. fluminea, Morton (1977) considered fluviali Chinae"; T. fluviatilis "in ilumine emporium Can C. leana to be a júnior synonym of C. fluminea, while C. tón Chinae praeterlabente". Since then, many living species fluviatilis was previously placed into synonymy with C. of Corbicula Mühlfeldt, 1811, have been described in fluminea by Prashad (1929). Moreover, a thorough review by freshwater and estuarine habitáis from Southeast Asia, the Britton and Morton (1979) lead the authors to consider that Indian subcontinent, the Pacific islands, and the easternmost most Asiatic species previously described could be attributed part of Europe and África (McMahon, 1983). -

Bankia Setacea Class: Bivalvia, Heterodonta, Euheterodonta
Phylum: Mollusca Bankia setacea Class: Bivalvia, Heterodonta, Euheterodonta Order: Imparidentia, Myida The northwest or feathery shipworm Family: Pholadoidea, Teredinidae, Bankiinae Taxonomy: The original binomen for Bankia the presence of long siphons. Members of setacea was Xylotrya setacea, described by the family Teredinidae are modified for and Tryon in 1863 (Turner 1966). William Leach distiguished by a wood-boring mode of life described several molluscan genera, includ- (Sipe et al. 2000), pallets at the siphon tips ing Xylotrya, but how his descriptions were (see Plate 394C, Coan and Valentich-Scott interpreted varied. Although Menke be- 2007) and distinct anterior shell indentation. lieved Xylotrya to be a member of the Phola- They are commonly called shipworms (though didae, Gray understood it as a member of they are not worms at all!) and bore into many the Terdinidae and synonyimized it with the wooden structures. The common name ship- genus Bankia, a genus designated by the worm is based on their vermiform morphology latter author in 1842. Most authors refer to and a shell that only covers the anterior body Bankia setacea (e.g. Kozloff 1993; Sipe et (Ricketts and Calvin 1952; see images in al. 2000; Coan and Valentich-Scott 2007; Turner 1966). Betcher et al. 2012; Borges et al. 2012; Da- Body: Bizarrely modified bivalve with re- vidson and de Rivera 2012), although one duced, sub-globular body. For internal anato- recent paper sites Xylotrya setacea (Siddall my, see Fig. 1, Canadian…; Fig. 1 Betcher et et al. 2009). Two additional known syno- al. 2012. nyms exist currently, including Bankia Color: osumiensis, B. -

(Corbicula Fluminea) En Limousin. Synthèse Des Connaissances Et
La corbicule asiatique (Corbicula fluminea) en Limousin. Synthèse des connaissances et répartition régionale en 2014. David Naudon, Groupe Mulette Limousin Centre nature La Loutre Domaine des Vaseix, 87430 Verneuil sur Vienne [email protected] 05/55/48/07/88 Introduction : Près d'Aixe-sur-Vienne, à la fin des années 1990, quelques promeneurs et pêcheurs avaient remarqué la présence de petits coquillages, de 2 à 4 cm de diamètre, inconnus jusqu'alors sur la Vienne. Se penchant sur la question, quelques naturalistes confirmaient bien qu'il s'agissait d'un nouveau mollusque pour notre région : la corbicule. Ce drôle de petit coquillage, dont la forme rappelle celle des coques ou des palourdes, nous vient d'Asie où il est largement répandu. Corbicula fluminea, puisque c’est son nom, est l'une des espèces les plus envahissantes dans les écosystèmes aquatiques d'eau douce (Sousa, R. et al. 2008). Sa croissance rapide, sa maturité sexuelle précoce, sa forte capacité de reproduction, sa plasticité écologique et les bénéfices qu’elle tire des activités humaines lui promettent de beaux jours ! Capable de s'adapter dans de nombreux cours d'eau, cette « palourde » asiatique a rapidement conquis de nouveaux territoires. Aujourd'hui la corbicule est présente sur l’ensemble des grandes rivières limousines où elle continue sa progression. Cette note fait le point sur les connaissances générales sur cette espèce et sur sa répartition en Limousin à ce jour. La partie concernant les connaissances générales de la corbicule est largement empruntée à différents travaux, notamment ceux de Sylvain Vrignaud que je remercie ici pour sa large contribution et son aide précieuse. -

Alabama Map Turtle
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project ofEmydidae the IUCN/SSC — TortoiseGraptemys and Freshwater pulchra Turtle Specialist Group 072.1 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson, and R.A. Mittermeier, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.072.pulchra.v1.2014 © 2014 by Chelonian Research Foundation • Published 6 January 2014 Graptemys pulchra Baur 1893 – Alabama Map Turtle JEFFREY E. LOVICH 1, JAMES C. GODWIN 2, AND C.J. MCCOY 3 1U.S. Geological Survey, Southwest Biological Science Center, 2255 North Gemini Drive MS-9394, Flagstaff, Arizona 86001 USA [[email protected]]; 2Alabama Natural Heritage Program, Environmental Institute, 1090 S. Donahue Drive, Auburn University, Alabama 36849 USA [[email protected]]; 3Deceased; formerly Carnegie Museum of Natural History, 4400 Forbes Ave., Pittsburgh, Pennsylvania 15213 USA SUMMARY . – The Alabama Map Turtle, Graptemys pulchra (Family Emydidae), is a moderately large riverine species endemic to the Mobile Bay drainage system of Alabama, Georgia, and Missis- sippi. Sexual size dimorphism is pronounced, with adult females (carapace length [CL] to 273 mm) attaining more than twice the size of adult males (CL to 117 mm). The species is an inhabitant of relatively large, swift creeks and rivers, often with wide sandbars. Stream sections open to the sun and with abundant basking sites in the form of logs and brush are preferred. Six to seven clutches of 4–7 eggs are laid each year on river sandbars. Although the species is locally abundant, popula- tions are threatened by habitat destruction, declines in their prey base, commercial collection, and vandalism. -

Caridina Variabilirostris (Crustacea: Decapoda: Atyidae), a New Species
Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia) Valentin de Mazancourt, Gerard Marquet, Philippe Keith To cite this version: Valentin de Mazancourt, Gerard Marquet, Philippe Keith. Caridina variabilirostris (Crustacea: De- capoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia). European Journal of Taxonomy, Consortium of European Natural History Museums, 2018, pp.1-16. 10.5852/ejt.2018.453. hal-02171169 HAL Id: hal-02171169 https://hal.archives-ouvertes.fr/hal-02171169 Submitted on 2 Jul 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. European Journal of Taxonomy 453: 1–16 ISSN 2118-9773 https://doi.org/10.5852/ejt.2018.453 www.europeanjournaloftaxonomy.eu 2018 · Mazancourt V. de et al. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:445C8252-5FA2-49AA-AB89-E1C2DDEBEE7F Caridina variabilirostris (Crustacea: Decapoda: Atyidae), a new species of freshwater shrimp from Pohnpei (Micronesia) Valentin de MAZANCOURT 1,*, Gerard MARQUET 2 & Philippe KEITH 3 1,2,3 Muséum national d’Histoire naturelle, Département Adaptations du Vivant, UMR 7208, CP026, 57, rue Cuvier, 75231 Paris, Cedex 05, France.