A New Multiple Trauma Model of the Mouse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Femoral Shaft Fractures Andrew Chen, MD University of North Carolina
Femoral Shaft Fractures Andrew Chen, MD University of North Carolina Core Curriculum V5 Disclosure All figures belong to Andrew Chen, MD unless otherwise indicated Core Curriculum V5 Objectives • Review initial management of femoral shaft fractures and possible concomitant injuries • Discuss multiple options with intramedullary nailing • Antegrade/retrograde • Starting point • Reaming • Patient positioning • Understand commonly associated complications Core Curriculum V5 Femoral Shaft Fractures • Bimodal distribution • Young patients after high-energy trauma • Elderly patients after falls from standing secondary to osteopenia/osteoporosis • MVC, MCC, pedestrian struck, fall from height, and gunshot wounds most common mechanisms • Intramedullary nail as “gold standard” treatment, which has continued to evolve since introduction by Gerhard Küntscher around World War II Core Curriculum V5 Anatomy • Largest and strongest bone in body • Anterior bow with radius of curvature ~120 cm1 • Blood supply from primary nutrient vessel through linea aspera and small periosteal vessels • Deformity pattern dependent on attached musculature • Proximal fragment • Flexed (gluteus medius/minimus on greater trochanter) • Abducted (iliopsoas on lesser trochanter) • Distal fragment • Varus (adductors inserting on medial aspect distal femur) • Extension (gastrocnemius attaching on distal aspect of posterior femur) Courtesy of Rockwood and Green’s Fracture in Adults2 Core Curriculum V5 Femur Fracture Classification: AO/OTA • Bone Segment 32 • Type A • Simple • -

Unusual Case of Primary Spontaneous Hemopneumothorax in a Young Man with Atypical Tension Pneumothorax: a Case Report Youwen Chen* and Zhijian Guo
Chen and Guo Journal of Medical Case Reports (2018) 12:188 https://doi.org/10.1186/s13256-018-1732-x CASE REPORT Open Access Unusual case of primary spontaneous hemopneumothorax in a young man with atypical tension pneumothorax: a case report Youwen Chen* and Zhijian Guo Abstract Background: Spontaneous life-threatening hemopneumothorax is an atypical but treatable entity of unexpected circulatory collapse in young patients, affecting 0.5–11.6% of patients with primary spontaneous pneumothorax. Spontaneous pneumothorax is a well-documented disorder with a classic clinical presentation of acute onset chest pain and shortness of breath. This disorder might be complicated by the development of hemopneumothorax or tension pneumothorax. Case presentation: A 23-year-old Asian man was referred to the emergency room of Xiamen Chang Gung Memorial Hospital with a 1-day history of right-sided chest pain that had been aggravated for 1 hour. A physical examination revealed a young man who was awake and alert but in mild to moderate painful distress. His vital parameters were relatively stable at first. The examining physician noted slight tenderness along the right posterolateral chest wall along the eighth and tenth ribs. Primary spontaneous pneumothorax was considered, and a standing chest X-ray confirmed the diagnosis. A right thoracostomy tube was immediately placed under sterile conditions, and he was referred to the respiratory service. While in the respiratory department, approximately 420 mL of blood was drained from the thoracostomy tube over 15 minutes. Our patient developed obvious hemodynamic instability with hypovolemic shock and was subsequently admitted to the cardiothoracic surgical ward after fluid resuscitation. -

Rare Combination of Ipsilateral Acetabular Fracture-Dislocation and Pertrochanteric Fracture
A Case Report & Literature Review Rare Combination of Ipsilateral Acetabular Fracture-Dislocation and Pertrochanteric Fracture Kevin M. Kuhn, CDR, MC, USN, John A. Boudreau, MD, and J. Tracy Watson, MD oral fractures. Other case reports have described acetabular Abstract fracture-dislocations associated with femoral neck fractures.1-3 Acetabular fracture-dislocations are severe This case report describes an acetabular fracture-dislocation injuries that require urgent closed reduction associated with an ipsilateral pertrochanteric fracture and sub- of the hip and often require surgery to restore trochanteric extension. hip stability. Other authors have described We propose a staged treatment strategy consisting of early acetabular fracture-dislocations associated minimally invasive reduction of the hip and delayed reduction with femoral neck fractures, but to our knowl- and fixation of the fractures. This strategy may be useful in edge, this case report is the first to describe an managing a polytraumatized patient who may not be stable acetabular fracture-dislocation in association enough to undergo early definitive management, or a patient with an ipsilateral pertrochanteric fracture and who requires prolonged transfer to receive definitive care. subtrochanteric extension. The patient provided written informed consent for print The polytraumatized patient initially was not and electronic publication of this case report. stable enough for prolonged surgery. Through a 3-cm anterolateral hip incision, a 5-mmAJO Schanz Case Report screw was introduced percutaneously into the A 44-year-old man was involved in a head-on motor vehicle femoral head through the primary fracture site collision at highway speed. He was taken to a local hospital, under fluoroscopic guidance. -

Treatment of Common Hip Fractures: Evidence Report/Technology
This report is based on research conducted by the Minnesota Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. HHSA 290 2007 10064 1). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help clinicians, employers, policymakers, and others make informed decisions about the provision of health care services. This report is intended as a reference and not as a substitute for clinical judgment. This report may be used, in whole or in part, as the basis for the development of clinical practice guidelines and other quality enhancement tools, or as a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. Evidence Report/Technology Assessment Number 184 Treatment of Common Hip Fractures Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA 290 2007 10064 1 Prepared by: Minnesota Evidence-based Practice Center, Minneapolis, Minnesota Investigators Mary Butler, Ph.D., M.B.A. Mary Forte, D.C. Robert L. Kane, M.D. Siddharth Joglekar, M.D. Susan J. Duval, Ph.D. Marc Swiontkowski, M.D. -

Clinical Study Outcome of Concurrent Occult Hemothorax and Pneumothorax in Trauma Patients Who Required Assisted Ventilation
Hindawi Publishing Corporation Emergency Medicine International Volume 2015, Article ID 859130, 6 pages http://dx.doi.org/10.1155/2015/859130 Clinical Study Outcome of Concurrent Occult Hemothorax and Pneumothorax in Trauma Patients Who Required Assisted Ventilation Ismail Mahmood,1 Zainab Tawfeek,2 Ayman El-Menyar,3,4,5 Ahmad Zarour,1 Ibrahim Afifi,1 Suresh Kumar,1 Ruben Peralta,1 Rifat Latifi,1 and Hassan Al-Thani1 1 Department of Surgery, Section of Trauma Surgery, Hamad General Hospital, P.O. Box 3050, Doha, Qatar 2Department of Emergency, Hamad Medical Corporation, P.O. Box 3050, Doha, Qatar 3Clinical Research, Section of Trauma Surgery, Hamad General Hospital, Doha, Qatar 4ClinicalMedicine,WeillCornellMedicalSchool,P.O.Box24144,Doha,Qatar 5Internal Medicine, Ahmed Maher Teaching Hospital, Cairo, Egypt Correspondence should be addressed to Ismail Mahmood; [email protected] Received 26 October 2014; Accepted 3 February 2015 Academic Editor: Seiji Morita Copyright © 2015 Ismail Mahmood et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. The management and outcomes of occult hemopneumothorax in blunt trauma patients who required mechanical ventilation are not well studied. We aimed to study patients with occult hemopneumothorax on mechanical ventilation who could be carefully managed without tube thoracostomy. Methods. Chest trauma patients with occult hemopneumothorax who were on mechanical ventilation were prospectively evaluated. The presence of hemopneumothorax was confirmed by CT scanning. Hospital length of stay, complications, and outcome were recorded. Results.Atotalof56chesttraumapatientswithoccult hemopneumothorax who were on ventilatory support were included with a mean age of 36 ± 13 years. -

Femoral Shaft Fracture Fixation and Chest Injury After Polytrauma
This is an enhanced PDF from The Journal of Bone and Joint Surgery The PDF of the article you requested follows this cover page. Femoral Shaft Fracture Fixation and Chest Injury After Polytrauma Lawrence B. Bone and Peter Giannoudis J Bone Joint Surg Am. 2011;93:311-317. doi:10.2106/JBJS.J.00334 This information is current as of January 25, 2011 Reprints and Permissions Click here to order reprints or request permission to use material from this article, or locate the article citation on jbjs.org and click on the [Reprints and Permissions] link. Publisher Information The Journal of Bone and Joint Surgery 20 Pickering Street, Needham, MA 02492-3157 www.jbjs.org 311 COPYRIGHT Ó 2011 BY THE JOURNAL OF BONE AND JOINT SURGERY,INCORPORATED Current Concepts Review Femoral Shaft Fracture Fixation and Chest Injury After Polytrauma By Lawrence B. Bone, MD, and Peter Giannoudis, MD, FRCS Thirty years ago, the standard of care for the multiply injured tients with multiple injuries, defined as an ISS of ‡18, and patient with fractures was placement of the fractured limb in a patients with essentially an isolated femoral fracture and an splint or skeletal traction, until the patient was considered stable ISS of <18. Pulmonary complications consisting of ARDS, enough to undergo surgery for fracture fixation1. This led to a pulmonary dysfunction, fat emboli, pulmonary emboli, and number of complications2, such as adult respiratory distress pneumonia were present in 38% (fourteen) of thirty-seven syndrome (ARDS), infection, pneumonia, malunion, non- patients in the late fixation/multiple injuries group and 4% union, and death, particularly when the patient had a high (two) of forty-six in the early fixation/multiple injuries group; Injury Severity Score (ISS)3. -

Presumptive Antibiotics in Tube Thoracostomy for Traumatic
Trauma Surg Acute Care Open: first published as 10.1136/tsaco-2019-000356 on 4 November 2019. Downloaded from Open access Plenary paper Presumptive antibiotics in tube thoracostomy for traumatic hemopneumothorax: a prospective, Multicenter American Association for the Surgery of Trauma Study Alan Cook ,1 Chengcheng Hu,2 Jeanette Ward,3 Susan Schultz,4 Forrest O’Dell Moore III,5 Geoffrey Funk,6 Jeremy Juern,7 David Turay,8 Salman Ahmad,9 Paola Pieri,10 Steven Allen,11 John Berne,12 for the AAST Antibiotics in Tube Thoracostomy Study Group For numbered affiliations see ABSTRact a hemothorax, pneumothorax, or hemopneu- end of article. Background Thoracic injuries are common in trauma. mothorax (HPTX).1–4 Although no statistics are Approximately one- third will develop a pneumothorax, available for the number of post- traumatic tube Correspondence to hemothorax, or hemopneumothorax (HPTX), usually with thoracostomies (TT) performed in the USA annu- Dr Forrest O’Dell Moore III, John Peter Smith Healthcare Network, concomitant rib fractures. Tube thoracostomy (TT) is the ally, this commonly performed procedure remains Fort Worth, TX 76104, USA; standard of care for these conditions, though TTs expose the first- line treatment for drainage of the pleural fmoore@ jpshealth. org the patient to the risk of infectious complications. The cavity. controversy regarding antibiotic prophylaxis at the time It is well documented that TTs placed in the Presented at the American trauma setting are associated with increased Association for the Surgery of of TT placement remains unresolved. This multicenter 5 6 Trauma 77th Annual Meeting, study sought to reconcile divergent evidence regarding hospital length of stay (LOS), morbidity, and cost. -

Emergency Department Evaluation and Management of Blunt Chest
June 2016 Emergency Department Volume 18, Number 6 Authors Evaluation And Management Of Eric J. Morley, MD, MS Associate Professor of Clinical Emergency Medicine, Associate Residency Director, Department of Emergency Medicine, Stony Brook Blunt Chest And Lung Trauma Medicine, Stony Brook, NY Scott Johnson, MD Associate Professor of Clinical Emergency Medicine, Residency Abstract Director, Department of Emergency Medicine, Stony Brook Medicine, Stony Brook, NY The majority of blunt chest injuries are minor contusions or Evan Leibner, MD, PhD Department of Emergency Medicine, Stony Brook Medicine, Stony abrasions; however, life-threatening injuries, including tension Brook, NY pneumothorax, hemothorax, and aortic rupture can occur and Jawad Shahid, MD must be recognized early. This review focuses on the diagnosis, Department of Emergency Medicine, Stony Brook Medicine, Stony management, and disposition of patients with blunt injuries to Brook, NY the ribs and lung. Utilization of decision rules for chest x-ray and Peer Reviewers computed tomography are discussed, along with the emerging Ram Parekh, MD role of bedside lung ultrasonography. Management controversies Assistant Clinical Professor, Emergency Department, Elmhurst Hospital presented include the limitations of needle thoracostomy us- Center, Icahn School of Medicine at Mount Sinai, New York, NY Christopher R. Tainter, MD, RDMS ing standard needle, chest tube placement, and chest tube size. Assistant Clinical Professor, Department of Emergency Medicine, Finally, a discussion is provided related to airway and ventilation Department of Anesthesiology, Division of Critical Care, University of management to assist in the timing and type of interventions California San Diego, San Diego, CA needed to maintain oxygenation. Prior to beginning this activity, see “Physician CME Information” on the back page. -

Pulmonary Dysfunction in Patients with Femoral Shaft Fracture Treated with Intramedullary Nailing by BRENT L
COPYRIGHT © 2001 BY THE JOURNAL OF BONE AND JOINT SURGERY, INCORPORATED Pulmonary Dysfunction in Patients with Femoral Shaft Fracture Treated with Intramedullary Nailing BY BRENT L. NORRIS, MD, W. CHRISTOPHER PATTON, MD, JOSEPH N. RUDD JR., BSN, PHD, COLLEEN M. SCHMITT, MD, MHS, AND JEFFREY A. KLINE, MD Investigation performed at the University of Tennessee College of Medicine, Chattanooga, Tennessee, and the Carolinas Medical Center, Charlotte, North Carolina Background: This study was undertaken to determine whether alveolar dead space increases during intramedul- lary nailing of femoral shaft fractures and whether alveolar dead space predicts postoperative pulmonary dysfunc- tion in patients undergoing intramedullary nailing of a femoral shaft fracture. Methods: All patients with a femoral shaft fracture were prospectively enrolled in the study unless there was evi- dence of acute myocardial infarction, shock, or heart failure. Arterial blood gases were measured at three consec- utive time-periods after induction of general anesthesia: before intramedullary nailing and ten and thirty minutes after intramedullary nailing. The end-tidal carbon-dioxide level, minute ventilation, positive end-expiratory pres- sure, and percent of inspired and expired inhalation agent were recorded simultaneously with the blood-gas mea- surement. Postoperatively, all subjects were monitored for evidence of pulmonary dysfunction, defined as the need for mechanical ventilation or supplemental oxygen (at a fraction of inspired oxygen of >40%) in the presence of clinical signs of a respiratory rate of >20 breaths/min or the use of accessory muscles of respiration. Results: Seventy-four patients with a total of eighty femoral shaft fractures completed the study. Fifty fractures (62.5%) underwent nailing after reaming, and thirty fractures (37.5%) underwent nailing with minimal or no ream- ing. -

Inclusion/Exclusion Criteria (Re-Formatted May 2013 Final Version Distributed 5 December 2013)
Scottish Trauma Audit Group (STAG) Inclusion/Exclusion criteria (Re-formatted May 2013 Final version distributed 5 December 2013) The decision to include a patient should be based on the following points : A. ALL TRAUMA PATIENTS AGED 13 AND OVER B. WHO FULFILL THE FOLLOWING LENGTH OF STAY CRITERIA DIRECT ADMISSIONS TRANSFERRED PATIENTS (IN/OUT) Trauma admissions whose length of stay Trauma patients transferred in/out of ED for is at least 3 days or more specialist care whose combined hospital stay at e.g. both sites is 3 days or more into ED 18 th discharged 21 st (include) into ED 18 th discharged 20 th (exclude) OR Trauma patients who die in hospital within 3 days of attendance (do not include patients who enter ED with no recordable obs and declared dead within 15 mins) OR Trauma patients managed in Resus who meet inclusion criteria C. AND WHOSE INJURIES MEET THE FOLLOWING CRITERIA: BODY REGION OR INCLUDED EXCLUDED SPECIFIC INJURY HEAD All brain or skull injuries Isolated minor head injury (no fracture and GCS>13) FACE Fractures documented as Fractures documented as simple or significant displacement, open, stable. compound or comminuted. All - Lefort fractures panfacial fractures Orbital Blowout fractures THORAX All patients Isolated superficial lacerations, contusions, puncture wounds/bites with no underlying injury. ABDOMEN All patients Isolated superficial lacerations, contusions, puncture wounds/bites with no underlying injury. SPINE All None PELVIS All Isolated pubic rami fracture in ≥65 years old FEMORAL FRACTURE All (open or closed) Subtrochenteric fracture treated as a hip fracture. /.. Revised format circulated 23 May 2013 Updated by LH 5 December 2013 Filed: STAG Trauma//Active/Training/AUDIT GUIDELINES / BODY REGION OR INCLUDED EXCLUDED SPECIFIC INJURY HIP FRACTURE or PUBIC All ≥65 years with hip fracture OR pubic RAMI FRACTURE rami fracture with one other isolated injury. -

ACR Appropriateness Criteria Acute Hip Pain-Suspected Fracture
Revised 2018 American College of Radiology ACR Appropriateness Criteria® Acute Hip Pain-Suspected Fracture Variant 1: Acute hip pain. Fall or minor trauma. Suspect fracture. Initial imaging. Procedure Appropriateness Category Relative Radiation Level Radiography hip Usually Appropriate ☢☢☢ Radiography pelvis Usually Appropriate ☢☢ Radiography pelvis and hips Usually Appropriate ☢☢☢ CT pelvis and hips with IV contrast Usually Not Appropriate ☢☢☢ CT pelvis and hips without and with IV Usually Not Appropriate contrast ☢☢☢☢ CT pelvis and hips without IV contrast Usually Not Appropriate ☢☢☢ MRI pelvis and affected hip without and Usually Not Appropriate with IV contrast O MRI pelvis and affected hip without IV Usually Not Appropriate contrast O Bone scan hips Usually Not Appropriate ☢☢☢ US hip Usually Not Appropriate O Variant 2: Acute hip pain. Fall or minor trauma. Negative radiographs. Suspect fracture. Next imaging study. Procedure Appropriateness Category Relative Radiation Level MRI pelvis and affected hip without IV Usually Appropriate contrast O CT pelvis and hips without IV contrast Usually Appropriate ☢☢☢ CT pelvis and hips with IV contrast Usually Not Appropriate ☢☢☢ CT pelvis and hips without and with IV Usually Not Appropriate contrast ☢☢☢☢ MRI pelvis and affected hip without and with Usually Not Appropriate IV contrast O Bone scan hips Usually Not Appropriate ☢☢☢ US hip Usually Not Appropriate O ACR Appropriateness Criteria® 1 Acute Hip Pain-Suspected Fracture Acute Hip Pain-Suspected Fracture Expert Panel on Musculoskeletal Imaging: Andrew B. Ross, MD, MPHa; Kenneth S. Lee, MD, MBAb; Eric Y. Chang, MDc; Behrang Amini, MD, PhDd; Jennifer K. Bussell, MDe; Tetyana Gorbachova, MDf; Alice S. Ha, MDg; Bharti Khurana, MDh; Alan Klitzke, MDi; Pekka A. -
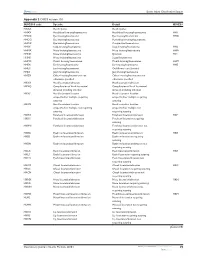
Appendix 2 OSICS Version 10.1 (Continued)
Dovepress Sports Injury Classification System Appendix 2 OSICS version 10.1 OSICS10 code Specific Detail OSICS9 HXXX Head injuries Head injuries HHXX Head/facial bruising/haematoma Head/facial bruising/haematoma HH1 HHOX Eye bruising/haematoma Eye bruising/haematoma HHO HHOO Eye bruising/haematoma Periorbital bruising/haematoma HHOC Eye bruising/haematoma Conjunctival haematoma HHSX Scalp bruising/haematoma Scalp bruising/haematoma HHS HHNX Nose bruising/haematoma Nose bruising/haematoma HHN HHNE Nose bruising/haematoma Epistaxis HV1 HHNS Nose bruising/haematoma Septal haematoma HHMX Mouth bruising/haematoma Mouth bruising/haematoma HHM HHEX Ear bruising/haematoma Ear bruising/haematoma HHE HHEC Ear bruising/haematoma Cauliflower ear (chronic) HHJX Jaw bruising/haematoma Jaw bruising/haematoma HHZX Other bruising/haematoma not Other bruising/haematoma not otherwise specified otherwise specified HKXX Head laceration/abrasion Head laceration/abrasion HKXQ Complication of head laceration/ Complication of head laceration/ abrasion including infection abrasion including infection HKXS Head laceration location Head laceration location unspecified/or multiple requiring unspecified/or multiple requiring suturing suturing HKXN Head laceration location Head laceration location unspecified/or multiple not requiring unspecified/or multiple not suturing requiring suturing HKHX Forehead laceration/abrasion Forehead laceration/abrasion HKF HKHS Forehead laceration/abrasion Forehead laceration requiring suturing HKHN Forehead laceration/abrasion Forehead