PLS Toolbox 4.0 Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Persistence in Algebraic Specifications
Persistence in Algebraic Specifications Freek Wiedijk Persistence in Algebraic Specifications Persistence in Algebraic Specifications Academisch Proefschrift ter verkrijging van de graad van doctor aan de Universiteit van Amsterdam, op gezag van de Rector Magnificus prof. dr. P.W.M. de Meijer, in het openbaar te verdedigen in de Aula der Universiteit (Oude Lutherse Kerk, ingang Singel 411, hoek Spui), op donderdag 12 december 1991 te 12.00 uur door Frederik Wiedijk geboren te Haarlem Promotores: prof. dr. P. Klint & prof. dr. J.A. Bergstra Faculteit Wiskunde en Informatica Het hier beschreven onderzoek werd mede mogelijk gemaakt door steun van de Ne- derlandse organisatie voor Wetenschappelijk Onderzoek (voorheen de nederlandse organisatie voor Zuiver-Wetenschappelijk Onderzoek) binnen project nummer 612- 317-013 getiteld Executeerbaar maken van algebraïsche specificaties. voor Jan Truijens Contents Contents Acknowledgements 1 Introduction 1 1.1 Motivation and general overview 2 1.2 Examples of erroneous specifications 13 1.3 Background and related work 22 2. Theory 25 2.1 Basic notions 27 2.1.1. Equational specifications 28 2.1.2 Term rewriting systems 29 2.2 Termination 30 2.2.1 Weak and strong termination 33 2.2.2 Path orderings 34 2.2.3 Decidability 40 2.3 Confluence 41 2.3.1 Weak and strong confluence 42 2.3.2 Overlapping 44 2.3.3 Decidability 45 2.4 Reduction strategies 46 2.5 Persistence 48 2.5.1 Weak and strong persistence 52 2.5.2 Term approximations and bases 54 2.5.3 Normal form analysis 56 2.5.4 Decidability 60 2.6 Primitive recursive -

Computations in Algebraic Geometry with Macaulay 2
Computations in algebraic geometry with Macaulay 2 Editors: D. Eisenbud, D. Grayson, M. Stillman, and B. Sturmfels Preface Systems of polynomial equations arise throughout mathematics, science, and engineering. Algebraic geometry provides powerful theoretical techniques for studying the qualitative and quantitative features of their solution sets. Re- cently developed algorithms have made theoretical aspects of the subject accessible to a broad range of mathematicians and scientists. The algorith- mic approach to the subject has two principal aims: developing new tools for research within mathematics, and providing new tools for modeling and solv- ing problems that arise in the sciences and engineering. A healthy synergy emerges, as new theorems yield new algorithms and emerging applications lead to new theoretical questions. This book presents algorithmic tools for algebraic geometry and experi- mental applications of them. It also introduces a software system in which the tools have been implemented and with which the experiments can be carried out. Macaulay 2 is a computer algebra system devoted to supporting research in algebraic geometry, commutative algebra, and their applications. The reader of this book will encounter Macaulay 2 in the context of concrete applications and practical computations in algebraic geometry. The expositions of the algorithmic tools presented here are designed to serve as a useful guide for those wishing to bring such tools to bear on their own problems. A wide range of mathematical scientists should find these expositions valuable. This includes both the users of other programs similar to Macaulay 2 (for example, Singular and CoCoA) and those who are not interested in explicit machine computations at all. -

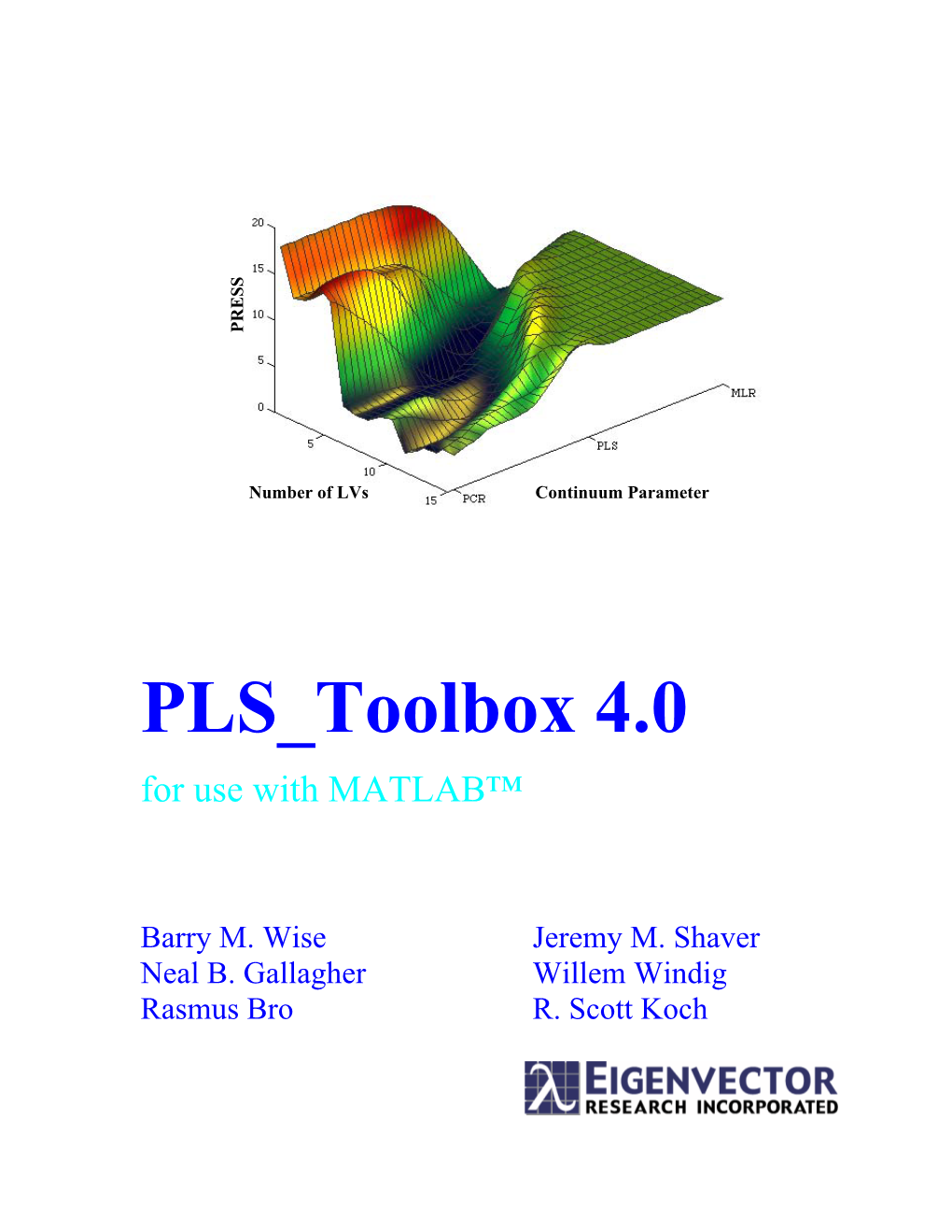
PLS Toolbox 7.0 Pltternf - Plots a 3D Ternary Diagram with Frequency of Occurrence
plotgui - Interactive data viewer. logdecay - Mean centers and variance scales a matrix using the log plttern - Plots a 2D ternary diagram. decay of the variable axis. PLS_Toolbox 7.0 pltternf - Plots a 3D ternary diagram with frequency of occurrence. lsq2top - Fits a polynomial to the top/(bottom) of data. querydb - Executes a query on a database defined by connection mdcheck - Missing Data Checker and infiller. Quick-Reference Card string. med2top - Fits a constant to top/(bottom) of data. Copyright © Eigenvector Research, 2012 reportwriter - Write a summary of the analysis including medcn - Median center scales matrix to median zero. associated figures to html/word/powerpoint. mncn - Scale matrix to mean zero. Help and Information rwb - Red white and blue color map. mscorr - Multiplicative scatter/signal correction (MSC). setpath - Modifies and saves current directory to the MATLAB normaliz - Normalize rows of matrix. helppls - Context related help on the PLS_Toolbox. search path. npreprocess - Preprocessing of multi-way arrays. readme - Release notes for Version 4.1 of PLS_Toolbox. snabsreadr - Reads Stellarnet ABS XY files. oscapp - Applies OSC model to new data. demos - Demo list for the PLS_Toolbox. spcreadr - Reads a Galactic SPC file. osccalc - Calculates orthogonal signal correction (OSC). evricompatibility - Tests for inter-product compatibility of trendtool - Univariate trend analysis tool. poissonscale - Perform Poisson scaling with scaling offset. Eigenvector toolboxes. vline - Adds vertical lines to figure at specified locations. polyinterp - Polynomial interpolation, smoothing, and evridebug - Checks the PLS_Toolbox installation for problems. writeasf - Writes AIT ASF files from a dataset object. differentiation. evridir - Locate and or create EVRI home directory. writecsv - Export a DataSet object to a comma-separated values preprocess - Selection and application of standard preprocessing evriinstall - Install Eigenvector Research Product. -

What Can Computer Algebraic Geometry Do Today?
What can computer Algebraic Geometry do today? Gregory G. Smith Wolfram Decker Mike Stillman 14 July 2015 Essential Questions ̭ What can be computed? ̭ What software is currently available? ̭ What would you like to compute? ̭ How should software advance your research? Basic Mathematical Types ̭ Polynomial Rings, Ideals, Modules, ̭ Varieties (affine, projective, toric, abstract), ̭ Sheaves, Divisors, Intersection Rings, ̭ Maps, Chain Complexes, Homology, ̭ Polyhedra, Graphs, Matroids, ̯ Established Geometric Tools ̭ Elimination, Blowups, Normalization, ̭ Rational maps, Working with divisors, ̭ Components, Parametrizing curves, ̭ Sheaf Cohomology, ঠ-modules, ̯ Emerging Geometric Tools ̭ Classification of singularities, ̭ Numerical algebraic geometry, ̭ ैक़௴Ь, Derived equivalences, ̭ Deformation theory,Positivity, ̯ Some Geometric Successes ̭ GEOGRAPHY OF SURFACES: exhibiting surfaces with given invariants ̭ BOIJ-SÖDERBERG: examples lead to new conjectures and theorems ̭ MODULI SPACES: computer aided proofs of unirationality Some Existing Software ̭ GAP,Macaulay2, SINGULAR, ̭ CoCoA, Magma, Sage, PARI, RISA/ASIR, ̭ Gfan, Polymake, Normaliz, 4ti2, ̭ Bertini, PHCpack, Schubert, Bergman, an idiosyncratic and incomplete list Effective Software ̭ USEABLE: documented examples ̭ MAINTAINABLE: includes tests, part of a larger distribution ̭ PUBLISHABLE: Journal of Software for Algebra and Geometry; www.j-sag.org ̭ CITATIONS: reference software Recent Developments in Singular Wolfram Decker Janko B¨ohm, Hans Sch¨onemann, Mathias Schulze Mohamed Barakat TU Kaiserslautern July 14, 2015 Wolfram Decker (TU-KL) Recent Developments in Singular July 14, 2015 1 / 24 commutative and non-commutative algebra, singularity theory, and with packages for convex and tropical geometry. It is free and open-source under the GNU General Public Licence. -

The Power of Pyramid Decomposition in Normaliz
Journal of Symbolic Computation 74 (2016) 513–536 Contents lists available at ScienceDirect Journal of Symbolic Computation www.elsevier.com/locate/jsc The power of pyramid decomposition in Normaliz Winfried Bruns a, Bogdan Ichim b, Christof Söger a a Universität Osnabrück, FB Mathematik/Informatik, 49069 Osnabrück, Germany b Simion Stoilow Institute of Mathematics of the Romanian Academy, Research Unit 5, C.P. 1-764, 010702 Bucharest, Romania a r t i c l e i n f o a b s t r a c t Article history: We describe the use of pyramid decomposition in Normaliz, a soft- Received 27 January 2014 ware tool for the computation of Hilbert bases and enumerative Accepted 26 August 2015 data of rational cones and affine monoids. Pyramid decomposition Available online 16 September 2015 in connection with efficient parallelization and streamlined evalua- tion of simplicial cones has enabled Normaliz to process triangula- MSC: ≈ · 11 52B20 tions of size 5 10 that arise in the computation of Ehrhart 13F20 series related to the theory of social choice. 14M25 © 2015 Elsevier Ltd. All rights reserved. 91B12 Keywords: Hilbert basis Ehrhart series Hilbert series Rational polytope Volume Triangulation Pyramid decomposition 1. Introduction Normaliz (Bruns et al., 2015)is a software tool for the computation of Hilbert bases and enumera- tive data of rational cones and affine monoids. In the 17 years of its existence it has found numerous applications; for example, in integer programming (Bogart et al., 2010), algebraic geometry (Craw et al., 2007), theoretical physics (Kappl et al., 2011), commutative algebra (Sturmfels and Welker, 2012) or elimination theory (Emiris et al., 2013). -

Normaliz 3.2.0 Contents
Normaliz 3.2.0 January 22, 2017 Winfried Bruns, Tim Römer, Richard Sieg and Christof Söger Normaliz 2 team member: Bogdan Ichim http://normaliz.uos.de https://github.com/Normaliz mailto:[email protected] Contents 1. Introduction 7 1.1. The objectives of Normaliz . .7 1.2. Platforms, implementation and access from other systems . .8 1.3. Major changes relative to version 3.1.1 . .9 1.4. Future extensions . .9 1.5. Download and installation . .9 2. Normaliz by examples 10 2.1. Terminology . 10 2.2. Practical preparations . 11 2.3. A cone in dimension 2 . 12 2.3.1. The Hilbert basis . 13 2.3.2. The cone by inequalities . 14 2.3.3. The interior . 15 2.4. A lattice polytope . 17 2.4.1. Only the lattice points . 20 2.5. A rational polytope . 20 2.5.1. The rational polytope by inequalities . 23 2.6. Magic squares . 24 2.6.1. With even corners . 27 2.6.2. The lattice as input . 30 1 2.7. Decomposition in a numerical semigroup . 30 2.8. A job for the dual algorithm . 31 2.9. A dull polyhedron . 32 2.9.1. Defining it by generators . 34 2.10. The Condorcet paradoxon . 35 2.10.1. Excluding ties . 36 2.10.2. At least one vote for every preference order . 37 2.11. Testing normality . 38 2.11.1. Computing just a witness . 39 2.12. Inhomogeneous congruences . 40 2.12.1. Lattice and offset . 41 2.12.2. Variation of the signs . 41 2.13. -

Program Synthesis
Program Synthesis Program Synthesis Sumit Gulwani Microsoft Research [email protected] Oleksandr Polozov University of Washington [email protected] Rishabh Singh Microsoft Research [email protected] Boston — Delft Foundations and Trends® in Programming Languages Published, sold and distributed by: now Publishers Inc. PO Box 1024 Hanover, MA 02339 United States Tel. +1-781-985-4510 www.nowpublishers.com [email protected] Outside North America: now Publishers Inc. PO Box 179 2600 AD Delft The Netherlands Tel. +31-6-51115274 The preferred citation for this publication is S. Gulwani, O. Polozov and R. Singh. Program Synthesis. Foundations and Trends® in Programming Languages, vol. 4, no. 1-2, pp. 1–119, 2017. ® This Foundations and Trends issue was typeset in LATEX using a class file designed by Neal Parikh. Printed on acid-free paper. ISBN: 978-1-68083-292-1 © 2017 S. Gulwani, O. Polozov and R. Singh All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, mechanical, photocopying, recording or otherwise, without prior written permission of the publishers. Photocopying. In the USA: This journal is registered at the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923. Authorization to photocopy items for in- ternal or personal use, or the internal or personal use of specific clients, is granted by now Publishers Inc for users registered with the Copyright Clearance Center (CCC). The ‘services’ for users can be found on the internet at: www.copyright.com For those organizations that have been granted a photocopy license, a separate system of payment has been arranged. -
![Arxiv:2010.08903V1 [Math.AC]](https://docslib.b-cdn.net/cover/5655/arxiv-2010-08903v1-math-ac-1565655.webp)
Arxiv:2010.08903V1 [Math.AC]
STANDARD PAIRS OF MONOMIAL IDEALS OVER NON-NORMAL AFFINE SEMIGROUPS IN SageMath BYEONGSU YU ABSTRACT. We present stdPairs.spyx,a SageMath library to compute standard pairs of a monomial ideal over a pointed (non-normal) affine semigroup ring. Moreover, stdPairs.spyx provides the associated prime ideals, the correspondingmultiplicities, and an irredundant irreducible primary decomposition of a monomial ideal. The library expands on the standardPairs func- tion on Macaulay2 over polynomial rings, and is based on algorithms from [6]. We also provide methods that allow the outputs from this library to be compatible with the Normaliz package of Macaulay2 and SageMath. 1. INTRODUCTION Affine semigroup rings are objects of many studies in combinatorial commutative algebra. The goal of this article is to present the SageMath library stdPairs.spyx, which systematizes computations for monomial ideals in affine semigroup rings. The algorithms implemented here are based on the notion of standard pairs, introduced for monomial ideals in polynomial rings by [8], and generalized to the semigroup ring case in [6]. Standard pairs are a combinatorial structure that contains information on primary and irreducible decompositions of monomial ideals, as well as multiplicities. One of the main contributions of [6] is that standard pairs and the associated algebraic concepts can be effectively computed over affine semigroup rings. The SageMath library stdPairs.spyx implements the algorithms of [6] to calculate standard pairs for monomial ideals in any pointed (non-normal) affine semigroup ring. This library can be regarded as a generalization of standardPairs function in Macaulay2 implemented by [4]. This library can be obtained via https://github.com/byeongsuyu/StdPairs. -
![Arxiv:2010.15331V2 [Math.AC] 19 Nov 2020 Rlqeto Nivratter.Ti Ril Ecie Significant a Describes R Article Action This Group Theory](https://docslib.b-cdn.net/cover/5111/arxiv-2010-15331v2-math-ac-19-nov-2020-rlqeto-nivratter-ti-ril-ecie-signi-cant-a-describes-r-article-action-this-group-theory-1935111.webp)
Arxiv:2010.15331V2 [Math.AC] 19 Nov 2020 Rlqeto Nivratter.Ti Ril Ecie Significant a Describes R Article Action This Group Theory
THE INVARIANTRING PACKAGE FOR MACAULAY2 LUIGI FERRARO, FEDERICO GALETTO, FRANCESCA GANDINI, HANG HUANG, MATTHEW MASTROENI, AND XIANGLONG NI Abstract. We describe a significant update to the existing InvariantRing package for Macaulay2. In addition to expanding and improving the methods of the existing package for actions of finite groups, the updated package adds func- tionality for computing invariants of diagonal actions of tori and finite abelian groups as well as invariants of arbitrary linearly reductive group actions. The implementation of the package has been completely overhauled with the aim of serving as a unified resource for invariant theory computations in Macaulay2. 1. Introduction Let G be a group acting linearly on an n-dimensional vector space V over a field K via v 7→ g · v for g ∈ G and v ∈ V . The action of G on V induces an action −1 of G on the polynomial ring K[V ]= K[x1,...,xn] by (g · f)(x)= f(g · x). By a classical result of Hilbert (see [DK15, 2.2.10]), the subring of invariant polynomials K[V ]G = {f ∈ K[V ] | g · f = f, ∀g ∈ G} is always a finitely generated K-algebra provided that G is linearly reductive and V is a rational representation of G.1 A linearly reductive group is a group that can be identified with a Zariski-closed subgroup of some general linear group GLn = GLn(K) and has good representation-theoretic properties while still being general enough to encompass all finite groups, all tori (K×)r, and all semisimple Lie groups (at least if char(K) = 0). -

An Introduction to Zeta Tobias Rossmann National University of Ireland, Galway
An introduction to Zeta Tobias Rossmann National University of Ireland, Galway Düsseldorf September 2018 Zeta (2014–) ... is a package for Sage (http://www.sagemath.org/) (which also relies on Singular, Normaliz, LattE, ...) ... provides methods for computing various types (ask, subobject, ...) of zeta functions in "fortunate cases" ... is freely available (http://www.maths.nuigalway.ie/~rossmann/Zeta/) ... implements techniques outlined here (https://doi.org/10.1007/978-3-319- 70566-8_25) Sage (2005–) is a free open-source mathematics software system licensed under the GPL. It builds on top of many existing open-source packages [...] Mission: Creating a viable free open source alternative to Magma, Maple, Mathematica and Matlab. Freely available (http://www.sagemath.org/) Based upon Python (https://www.python.org/) Includes GAP (https://www.gap-system.org/), Singular (http://www.singular.uni- kl.de/), ... Runs on Linux, Mac OS X, and Windows Command-line ("interactive shell") or browser-based ("notebook") interface Sage as a symbolic calculator −1 M (Z ) 1−p T Recall: the ask zeta function of 1 p is (1−T)2 . In [1]: var('p t') # create symbolic variables Z = (1-p^(-1)*t)/(1-t)^2 In [2]: Z.series(t,3) # power series expansion up to given order Out[2]: 1 + (-1/p + 2)*t + (-2/p + 3)*t^2 + Order(t^3) In [3]: Z(p=1) # substitution Out[3]: -1/(t - 1) In [4]: Z+1 # arithmetic Out[4]: -(t/p - 1)/(t - 1)^2 + 1 In [5]: _.simplify_full() Out[5]: (p*t^2 - (2*p + 1)*t + 2*p)/(p*t^2 - 2*p*t + p) In [6]: _.factor() Out[6]: (p*t^2 - 2*p*t + 2*p - t)/(p*(t - 1)^2) Installing Zeta Linux-only at the moment. -

Normaliz 2013-2016
NORMALIZ 2013–2016 WINFRIED BRUNS, RICHARD SIEG, AND CHRISTOF SOGER¨ ABSTRACT. In this article we describe mathematically relevant extensions to Normaliz that were added to it during the support by the DFG SPP “Algorithmische und Experi- mentelle Methoden in Algebra, Geometrie und Zahlentheorie”: nonpointed cones, ratio- nal polyhedra, homogeneous systems of parameters, bottom decomposition, class groups and systems of module generators of integral closures. 1. INTRODUCTION The software package Normaliz [12] has been developed by the algebra and discrete mathematics group at Osnabr¨uck since 1998. It is a tool for the computation of lattice points in rational polyhedra. Meanwhile it has been cited about 130 times in the literature FIGURE 1. Lattice points in a polyhedron (see [12]) with applications to algebraic geometry, commutative algebra, polytope theory, arXiv:1611.07965v2 [math.CO] 3 Mar 2017 integer programming, combinatorial topology, group theory, theoretical physics and other areas. There exist interfaces to the major computer algebra systems CoCoA [6], [2], GAP [20], Macaulay2 [19] and Singular [18] and to polymake [21], a comprehensive tool for the computation of polytopes. Normaliz is used by other packages, notably by Regina [17], a tool for the exploration of 3-manifolds, and by SecDec [7] in the computation of multiscale integrals. 2010 Mathematics Subject Classification. 52B20, 13F20, 14M25, 91B12. Key words and phrases. Hilbert basis, Hilbert series, rational cone, rational polyhedron, bottom decom- position, class group, triangulation, linear diophantine system. The second author was partially supported by the German Research Council DFG-GRK 1916 and a doctoral fellowship of the German Academic Exchange Service. 1 2 W.BRUNS,R.SIEG,ANDC.SOGER¨ During the second half of the SPP 1489 “Algorithmische und Experimentelle Metho- den in Algebra, Geometrie und Zahlentheorie” Normaliz was supported by the SPP. -

Fast Prototyping and Flexible C++ Library for Computations in Commutative Algebra
CoCoA and CoCoALib: Fast Prototyping and Flexible C++ Library for Computations in Commutative Algebra John Abbott Anna Maria Bigatti Institut fur¨ Mathematik Dipartimento di Matematica Universitat¨ Kassel Universita` degli Studi di Genova, Italy Kassel, Germany http://www.dima.unige.it/∼bigatti http://www.dima.unige.it/∼abbott Abstract—The CoCoA project began in 1987, and conducts (with two closely related applications being the interactive research into Computational Commutative Algebra (from which system and the compute server). This initial decision, together its name comes) with particular emphasis on Grobner¨ bases with the desire to help the software prosper, has many im- of ideals in multivariate polynomial rings, and related areas. A major output of the project is the CoCoA software, including the plications: e.g. designing a particularly clean interface for all CoCoA-5 interactive system and the CoCoALib C++ library. The functions with comprehensive documentation. This cleanliness software is open-source (GPL v.3), and under continual, active makes it easy to integrate CoCoALib into other software in a development. We give a summary of the features of the software trouble-free manner. likely to be relevant to the SC-Square community. Many computer algebra systems have a two-tier structure: I. INTRODUCTION a compiled kernel with interpreter, and an external library CoCoA-5 is a well-established Computer Algebra System of interpretable code for more advanced functions. Our aim specialized in operations on polynomial ideals, and a number with CoCoALib is different: we plan to have all functions of allied operations (e.g. factorization, and linear algebra). implemented directly in the C++ library so that all features It offers a dedicated, mathematician-friendly programming are available to programs which link to CoCoALib.