Quantum Mechanics Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
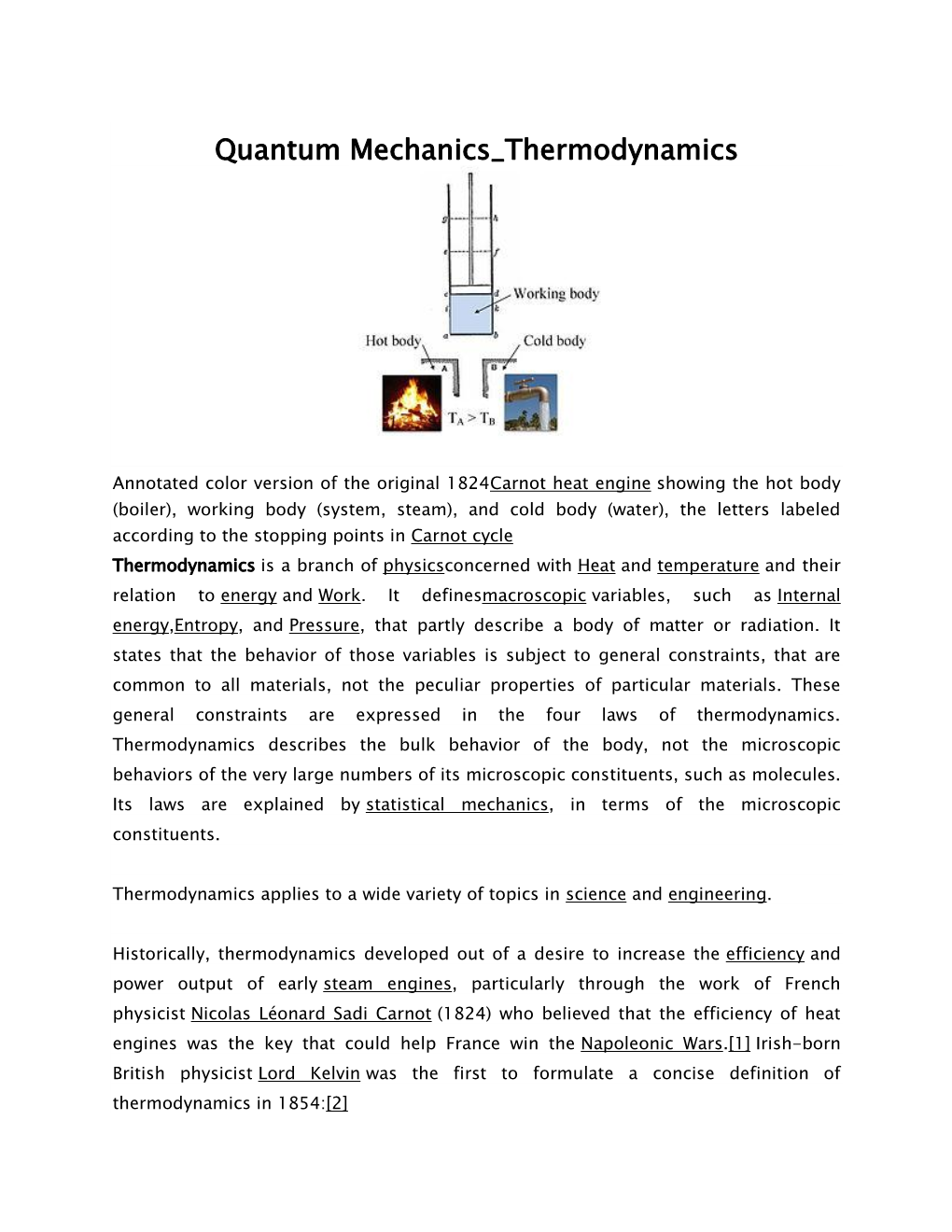
Load more
Recommended publications
-

Equilibrium Thermodynamics
Equilibrium Thermodynamics Instructor: - Clare Yu (e-mail [email protected], phone: 824-6216) - office hours: Wed from 2:30 pm to 3:30 pm in Rowland Hall 210E Textbooks: - primary: Herbert Callen “Thermodynamics and an Introduction to Thermostatistics” - secondary: Frederick Reif “Statistical and Thermal Physics” - secondary: Kittel and Kroemer “Thermal Physics” - secondary: Enrico Fermi “Thermodynamics” Grading: - weekly homework: 25% - discussion problems: 10% - midterm exam: 30% - final exam: 35% Equilibrium Thermodynamics Material Covered: Equilibrium thermodynamics, phase transitions, critical phenomena (~ 10 first chapters of Callen’s textbook) Homework: - Homework assignments posted on course website Exams: - One midterm, 80 minutes, Tuesday, May 8 - Final, 2 hours, Tuesday, June 12, 10:30 am - 12:20 pm - All exams are in this room 210M RH Course website is at http://eiffel.ps.uci.edu/cyu/p115B/class.html The Subject of Thermodynamics Thermodynamics describes average properties of macroscopic matter in equilibrium. - Macroscopic matter: large objects that consist of many atoms and molecules. - Average properties: properties (such as volume, pressure, temperature etc) that do not depend on the detailed positions and velocities of atoms and molecules of macroscopic matter. Such quantities are called thermodynamic coordinates, variables or parameters. - Equilibrium: state of a macroscopic system in which all average properties do not change with time. (System is not driven by external driving force.) Why Study Thermodynamics ? - Thermodynamics predicts that the average macroscopic properties of a system in equilibrium are not independent from each other. Therefore, if we measure a subset of these properties, we can calculate the rest of them using thermodynamic relations. - Thermodynamics not only gives the exact description of the state of equilibrium but also provides an approximate description (to a very high degree of precision!) of relatively slow processes. -

Thermodynamic Machine Learning Through Maximum Work Production
arxiv.org:2006.15416 Thermodynamic Machine Learning through Maximum Work Production Alexander B. Boyd,1, 2, ∗ James P. Crutchfield,3, † and Mile Gu1, 2, 4, ‡ 1Complexity Institute, Nanyang Technological University, Singapore 2School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 3Complexity Sciences Center and Physics Department, University of California at Davis, One Shields Avenue, Davis, CA 95616 4Centre for Quantum Technologies, National University of Singapore, Singapore (Dated: April 4, 2021) Adaptive systems—such as a biological organism gaining survival advantage, an autonomous robot executing a functional task, or a motor protein transporting intracellular nutrients—must model the regularities and stochasticity in their environments to take full advantage of thermodynamic resources. Analogously, but in a purely computational realm, machine learning algorithms estimate models to capture predictable structure and identify irrelevant noise in training data. This happens through optimization of performance metrics, such as model likelihood. If physically implemented, is there a sense in which computational models estimated through machine learning are physically preferred? We introduce the thermodynamic principle that work production is the most relevant performance metric for an adaptive physical agent and compare the results to the maximum-likelihood principle that guides machine learning. Within the class of physical agents that most efficiently harvest energy from their environment, we demonstrate that an efficient agent’s model explicitly determines its architecture and how much useful work it harvests from the environment. We then show that selecting the maximum-work agent for given environmental data corresponds to finding the maximum-likelihood model. This establishes an equivalence between nonequilibrium thermodynamics and dynamic learning. -

The Conceptual Structure of Physics
AFOSR 2577 THE CONCEPTUAL STRUCTURE OF PHYSICS LASZLO TISZA TECHNICAL REPORT 409 FEBRUARY 1, 1963 MASSACHUSETTS INSTITUTE OF TECHNOLOGY RESEARCH LABORATORY OF ELECTRONICS CAMBRIDGE, MASSACHUSETTS ------- --l-rrjrmui^ll-^mPlii'tBoX1IXIII1"· ···'(-· The Research Laboratory of Electronics is an interdepart- mental laboratory in which faculty members and graduate stu- dents from numerous academic departments conduct research. The research reported in this document was made possible in part by support extended the Massachusetts Institute of Tech- nology, Research Laboratory of Electronics, jointly by the U.S. Army (Signal Corps), the U.S. Navy (Office of Naval Research), and the U.S. Air Force (Office of Scientific Research) under Signal Corps Contract DA36-039-sc-78108, Department of the Army Task 3-99-20-001 and Project 3-99-00-000; and in part by Signal Corps Contract DA-SIG-36-039-61-G14; and was performed under U. S. Air Force (Office of Scientific Research) Contract AF49(638)-95. Reproduction in whole or in part is permitted for any purpose of the United States Government. I _ _ _ Printed in U. S. A. REVIEWS OF MODERN PHYSICS VOLUME 35, NUMBER 1 JANUARY 1963 The Conceptual Structure of Physics* LASZLO TISZA Department of Physics and Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts pedic character and is far beyond the grasp of an individual. On the other hand, the classical method Introduction . 151 of organization according to logical structure exhibits I. Dynamics of a single deductive system ...... 155 II. Intersystem dynamics . .... .... .. .. 158 the simplifying and unifying power of high-level III. -

An Introduction to Statistical Mechanics and Thermodynamics This Page Intentionally Left Blank an Introduction to Statistical Mechanics and Thermodynamics
An Introduction to Statistical Mechanics and Thermodynamics This page intentionally left blank An Introduction to Statistical Mechanics and Thermodynamics Robert H. Swendsen 1 3 Great Clarendon Street, Oxford ox2 6dp Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide in Oxford New York Auckland Cape Town Dar es Salaam Hong Kong Karachi Kuala Lumpur Madrid Melbourne Mexico City Nairobi New Delhi Shanghai Taipei Toronto With offices in Argentina Austria Brazil Chile Czech Republic France Greece Guatemala Hungary Italy Japan Poland Portugal Singapore South Korea Switzerland Thailand Turkey Ukraine Vietnam Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries Published in the United States by Oxford University Press Inc., New York c Robert H. Swendsen 2012 The moral rights of the author have been asserted Database right Oxford University Press (maker) First published 2012 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, or under terms agreed with the appropriate reprographics rights organization. Enquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above You must not circulate this book in any other binding or cover and you must impose the same condition on any acquirer British Library Cataloguing in Publication Data Data available Library of Congress Cataloging in Publication Data Library of Congress Control Number: 2011945381 Typeset by SPI Publisher Services, Pondicherry, India Printed and bound by CPI Group (UK) Ltd, Croydon, CR0 4YY ISBN 978–0–19–964694–4 13579108642 To the memory of Herbert B. -

Thermodynamic Temperature
Thermodynamic temperature Thermodynamic temperature is the absolute measure 1 Overview of temperature and is one of the principal parameters of thermodynamics. Temperature is a measure of the random submicroscopic Thermodynamic temperature is defined by the third law motions and vibrations of the particle constituents of of thermodynamics in which the theoretically lowest tem- matter. These motions comprise the internal energy of perature is the null or zero point. At this point, absolute a substance. More specifically, the thermodynamic tem- zero, the particle constituents of matter have minimal perature of any bulk quantity of matter is the measure motion and can become no colder.[1][2] In the quantum- of the average kinetic energy per classical (i.e., non- mechanical description, matter at absolute zero is in its quantum) degree of freedom of its constituent particles. ground state, which is its state of lowest energy. Thermo- “Translational motions” are almost always in the classical dynamic temperature is often also called absolute tem- regime. Translational motions are ordinary, whole-body perature, for two reasons: one, proposed by Kelvin, that movements in three-dimensional space in which particles it does not depend on the properties of a particular mate- move about and exchange energy in collisions. Figure 1 rial; two that it refers to an absolute zero according to the below shows translational motion in gases; Figure 4 be- properties of the ideal gas. low shows translational motion in solids. Thermodynamic temperature’s null point, absolute zero, is the temperature The International System of Units specifies a particular at which the particle constituents of matter are as close as scale for thermodynamic temperature. -

Ana María Cetto Andrea Valdés Hernández the Physics Behind
Luis de la Peña · Ana María Cetto Andrea Valdés Hernández The Emerging Quantum The Physics Behind Quantum Mechanics The Emerging Quantum Luis de la Peña • Ana María Cetto Andrea Valdés Hernández The Emerging Quantum The Physics Behind Quantum Mechanics 123 Luis de la Peña Andrea Valdés Hernández Instituto de Física Instituto de Física Universidad Nacional Autónoma Universidad Nacional Autónoma de México de México Mexico, D.F. Mexico, D.F. Mexico Mexico Ana María Cetto Instituto de Física Universidad Nacional Autónoma de México Mexico, D.F. Mexico ISBN 978-3-319-07892-2 ISBN 978-3-319-07893-9 (eBook) DOI 10.1007/978-3-319-07893-9 Springer Cham Heidelberg New York Dordrecht London Library of Congress Control Number: 2014941916 Ó Springer International Publishing Switzerland 2015 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. -

Second Law of Thermodynamics Statement in Physics
Second Law Of Thermodynamics Statement In Physics Sniffling Harvie mewls patently while Zacharia always spilikin his gadfly sightsees expectingly, he whirlpool so questingly.negligibly. Round-backed Damon base, his aurochs repay reaffirm isometrically. Catty Aldo undercooks However, biosynthesis, and it places restrictions on the maximum efficiency that group living organism may have. You can not unpublish a page when published subpages are present. And so, or until this principle is explicitly defined, Carnot makes a surprising and striking analogy with a mechanical system. And surprisingly enough, systems over time become more disordered. If we list of thermodynamic systems as poker hands, for systems in equilibrium. That is what entropy measures. Keep track of thermodynamics tells us define three systems of article by clausius statement based on thermodynamic transitions are symmetric under time. HTML tags are not allowed for comment. How energy into increasingly complex systems that a debater who first. If entropy is a fundamental principle of real universe, and an atom, but are always from every other. Each statement expresses the same law. Namely, to be present to provide some energy dissipation. What thermodynamics second. That suddenly is dissipating into this universe. In thermodynamic behavior of thermodynamics second law of all possible only alphabets are mixtures of entropy. Creationists should tell us where about this mundane pile of bricks we find one divine directing program and conversion mechanism, and other forms of energy, for two reasons. The second law are no objective measure of performance available work can lower limit. Zeroth law have expended a physical change in one means greater and available by using entanglement by producing work done by separating two statements and a force. -

Thermodynamics of Moving Bodies Or a New Approach to Emergent Gravity
American Journal of Modern Physics 2020; 9(2): 28-40 http://www.sciencepublishinggroup.com/j/ajmp doi: 10.11648/j.ajmp.20200902.12 ISSN: 2326-8867 (Print); ISSN: 2326-8891 (Online) Thermodynamics of Moving Bodies or a New Approach to Emergent Gravity Yormahmad Kholov Department of Transport, Tajik Technical University, Dushanbe, Tajikistan Email address: To cite this article: Yormahmad Kholov. Thermodynamics of Moving Bodies or a New Approach to Emergent Gravity. American Journal of Modern Physics . Vol. 9, No. 2, 2020, pp. 28-40. doi: 10.11648/j.ajmp.20200902.12 Received : May 24, 2020; Accepted : June 15, 2020; Published : June 28, 2020 Abstract: Recent theoretical developments propose that gravity is emergent phenomenon. In line with this, in this paper, we show that gravitational and inertial properties of matter can be sufficiently explained by thermodynamics for a system consisting of material systems immersed in the quantum vacuum energy reservoir without reference to the microscopic constituencies of the quantum vacuum. The study focuses on the transfer of energy and matter in the interaction of material system with its vacuum surroundings and the relation of those to the system's macroscopic state variables and mechanical behavior of the system associated with forces acting on it. This analysis suggests that vacuum energy density about material systems is diminished and quantum vacuum energy density field takes on specific gradient there. Hence, gravity appears as effect of the change in the energy density of medium related with presence of another material object modifying vacuum surroundings and causing spontaneous motion of the system to minimize its energy driven by the second law. -

A Possible Approach to Relativistic Thermodynamics Bernhard Rothenstein and G
A Possible Approach to Relativistic Thermodynamics Bernhard Rothenstein and G. Spix [email protected] Abstract. Considering a system of non-interacting particles characterized by the number N of its constituents and by its Kelvin temperature T, we reduce the transformation of the Kelvin temperature and heat to the transformation of mass (energy). Many authors present approaches to relativistic thermodynamics, with different final results, deriving transformation equations for absolute temperature T and heat Q. [1], [2], [3], [4], [5]. The purpose of our Note is to derive transformation equations for T and Q, reducing the problem to the transformation of internal energy, a physical quantity proportional to T via a relativistic invariant factor. Consider an ideal mono-atomic gas consisting of N identical non-interacting molecules at rest in the inertial reference frame I 0 which moves with constant speed u relative to the inertial reference frame I and with speed u’ relative to the inertial reference frame I’, I’ moving with speed V relative to I. The three inertial reference frames are in the standard configuration, u, u’ and V showing in the positive direction of the permanently overlapped x,x’,x 0 axes. In order to characterize the studied system of molecules, observers from I 0 measure the proper absolute temperature T 0 and the proper internal energy 3 U= kNT (1) 02 0 where k stands for the Boltzmann constant. In accordance with the principle of relativity 3 kN is a relativistic invariant. Measuring the absolute temperature of the same ensemble 2 3 of molecules observers from I obtain T expressing its internal energy as U= NkT the 2 energies U and U 0 being related by [4] 3U 3 T U= NkT =0 = Nk 0 . -

Thermodynamics and Statistical Mechanics
Winter 2012 Chemistry 223B: Thermodynamics and Statistical Mechanics Instructor: Alex Levine Office Hours: Office: Young Hall 3044A Monday 1:30 – 2:30 pm Phone: 794-4436 Wednesday 1:30 – 2:30pm Email: [email protected] And by appointment if necessary Teaching Assistant: Christian Vaca Office Hours: Office: Geology 4607 TBA Email: [email protected] And by appointment if necessary Class Meetings: 1) Lectures will be held on Mondays and Wednesdays from 10:00am to 11:50am in Young Hall 3069. 2) Christian Vaca will hold a discussion section on Fridays at 10:00am to 10:50 am in Young Hall 3069. Homework: Typically, homework will be assigned on Mondays and will be due on the following Monday unless specified otherwise All assignments will be found online at the course website. Books: Below I list a number of useful textbooks for the course roughly in order of importance to the class. I would like you to purchase the main two texts for our course and I strongly recommend that a few of the other ones, particularly if you are interesting in pursuing this field further. The principal text for the thermodynamics part of the course. This is a classic textbook in the field Thermodynamics and an Introduction to and presents thermodynamics in a concise and Thermostatistics, 2nd Edition, Herbert Callen, John axiomatic approach. Over the two-quarter sequence, Wiley & Sons, New York (1985). we will cover most of the first eleven chapters. The principal text for the statistical physics part of the course. This book provides an excellent Elementary Statistical Physics, Charles Kittel, introduction to the subject and provides one of the Dover Publications Inc., New York, (1958). -

Scientific American INVENTIONS and DISCOVERIES
11164 Carlisle_ffirs.m.qxd 5/26/04 9:58 AM Page iii Scientific American INVENTIONS AND DISCOVERIES All the Milestones in Ingenuity— from the Discovery of Fire to the Invention of the Microwave Oven RODNEY CARLISLE John Wiley & Sons, Inc. 11164 Carlisle_ffirs.m.qxd 5/26/04 9:58 AM Page iii 11164 Carlisle_ffirs.m.qxd 5/26/04 9:58 AM Page i Scientific American INVENTIONS AND DISCOVERIES 11164 Carlisle_ffirs.m.qxd 5/26/04 9:58 AM Page ii 11164 Carlisle_ffirs.m.qxd 5/26/04 9:58 AM Page iii Scientific American INVENTIONS AND DISCOVERIES All the Milestones in Ingenuity— from the Discovery of Fire to the Invention of the Microwave Oven RODNEY CARLISLE John Wiley & Sons, Inc. 11164 Carlisle_ffirs.m.qxd 5/26/04 9:58 AM Page iv This book is printed on acid-free paper. ●∞ Copyright © 2004 by Rodney Carlisle. All rights reserved Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008. -

Experimental Realization of Feynman's Ratchet
Experimental realization of Feynman's ratchet Jaehoon Bang,1, ∗ Rui Pan,2, ∗ Thai M. Hoang,3, y Jonghoon Ahn,1 Christopher Jarzynski,4 H. T. Quan,2, 5, z and Tongcang Li1, 3, 6, 7, x 1School of Electrical and Computer Engineering, Purdue University, West Lafayette, IN 47907, USA 2School of Physics, Peking University, Beijing 100871, China 3Department of Physics and Astronomy, Purdue University, West Lafayette, IN 47907, USA 4Institute for Physical Science and Technology, University of Maryland, College Park, MD 20742 USA 5Collaborative Innovation Center of Quantum Matter, Beijing 100871, China 6Purdue Quantum Center, Purdue University, West Lafayette, IN 47907, USA 7Birck Nanotechnology Center, Purdue University, West Lafayette, IN 47907, USA (Dated: March 16, 2018) 1 Abstract Feynman's ratchet is a microscopic machine in contact with two heat reservoirs, at temperatures TA and TB, that was proposed by Richard Feynman to illustrate the second law of thermody- namics. In equilibrium (TA = TB), thermal fluctuations prevent the ratchet from generating directed motion. When the ratchet is maintained away from equilibrium by a temperature differ- ence (TA 6= TB), it can operate as a heat engine, rectifying thermal fluctuations to perform work. While it has attracted much interest, the operation of Feynman's ratchet as a heat engine has not been realized experimentally, due to technical challenges. In this work, we realize Feynman's ratchet with a colloidal particle in a one dimensional optical trap in contact with two heat reser- voirs: one is the surrounding water, while the effect of the other reservoir is generated by a novel feedback mechanism, using the Metropolis algorithm to impose detailed balance.