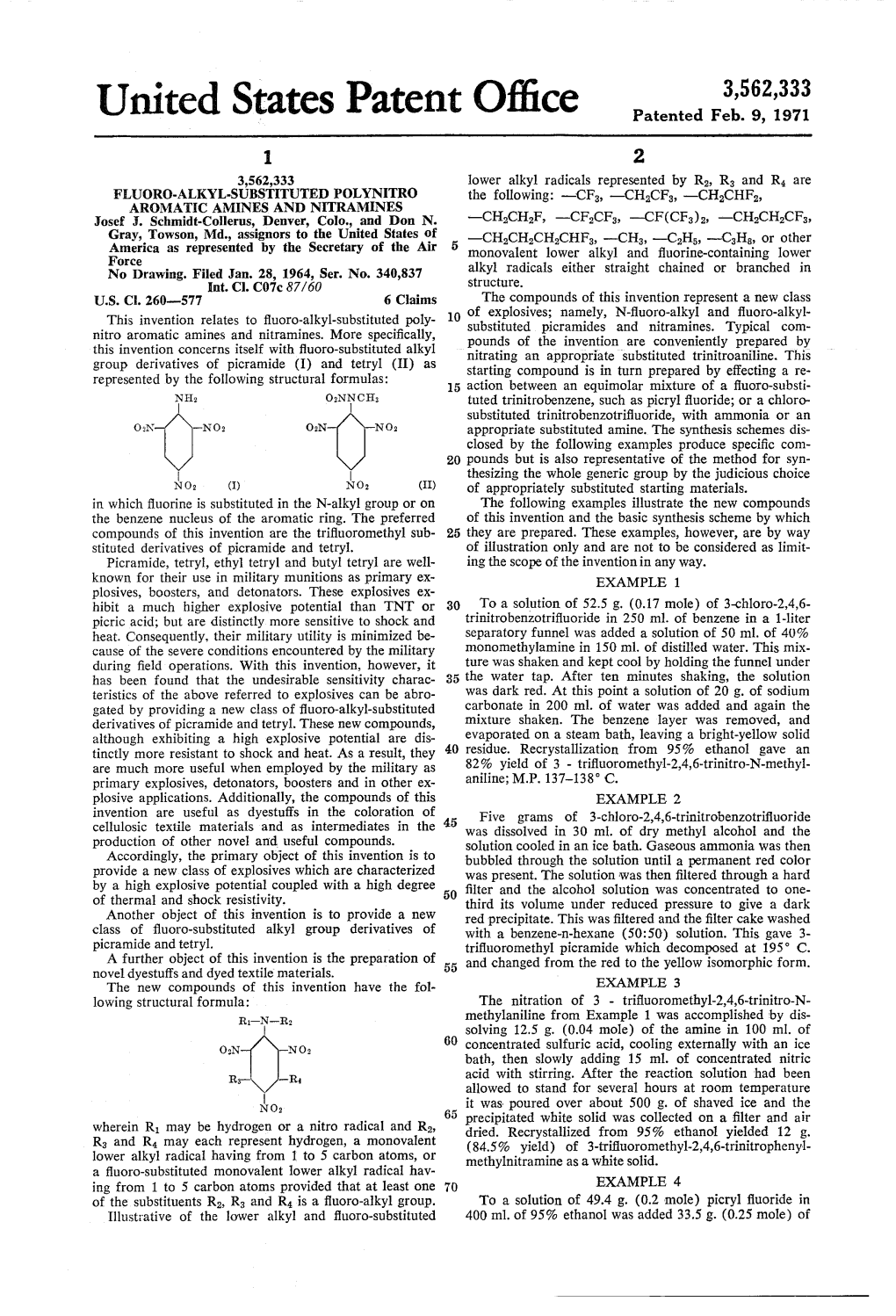
United States Patent Office Patented Feb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Field-Based Analytical Methods for Explosive Compounds
Field-Based Analytical Methods for Explosive Compounds Dr. Thomas F. Jenkins Marianne E. Walsh USA Engineer Research and Development Center– Cold Regions Research and Engineering Laboratory 72 Lyme Road, Hanover NH 03755 603-646-4385 (FAX-4785) [email protected] [email protected] 1 1 Outline of Presentation • Important properties of nitroaromatic (TNT) and nitramine (RDX) explosives • Accepted laboratory methods for explosives chemicals • Detection criteria for explosives-related chemicals • Why should you consider using on-site methods? • Sampling considerations for explosives in soil and water • Verified methods for on-site determination of explosives in soil and water • Advantages / disadvantages of various on-site methods 2 Overview of topics to be covered in the presentation. 2 Nitroaromatic Nitramine (TNT) (RDX) NO 2 CH3 N O N 2 NO2 N N O N 2 NO 2 NO 2 H C-O-NO 2 2 Nitrate Ester HC-O-NO (NG) 2 H 2 C-O-NO2 3 3 ***Safety*** • Chunks of high explosives often found at contaminated sites • Concentrations of TNT or RDX in soil greater than 12% are reactive (can propagate a detonation)* • Neither chunks nor soil with concentrations of TNT and RDX greater than 10% can be shipped off site using normal shipping procedures *Kristoff et al. 1987 4 The most important property of all is the ability of these compounds to detonate if they are subjected to the right type of stimulus (spark, shock). This is one of the major reasons why on-site analysis is so important for explosives. Kristoff, F.T., T.W. -

TWGFEX Suggested Guide for Explosive Analysis Training
Suggested Guide for Explosive Analysis Training This is a suggested guideline for the training of an examiner in the field of explosive analysis by an appropriately qualified explosives examiner/analyst. If an appropriate instructor is unavailable, the corresponding sections of the training should be sought externally. This training guide may be modified to fit an agency’s training requirements and goals. All training must be conducted following proper safety procedures as prescribed by the appropriate agency/organization guidelines, as well as all applicable laws/regulations. When practical and available, coordination with local bomb squads is highly recommended. All training must include proper documentation upon completion of each section/module. The trainee may not have to complete all sections. The needs and available instrumentation of the agency will dictate the individual training program selected from the following guidelines. However, the trainee needs to be aware of other analytical methods that are utilized elsewhere. Methods of instruction may include instructions by trainer, self study and/or online guides and/or external instruction. Method of evaluation may include oral, written and/or practical exercise(s). Suggested readings may be found for each section in the accompanying bibliography. I. Introduction Introduction to Explosives Objectives: Upon completion of this unit the student will be able to: 1. Describe the historical development of explosives. (i.e. black powder, TNT, smokeless powder, nitroglycerin, pyrotechnics, etc.) 2. Describe the different types of explosives and how they can be classified. Examples of the different classification systems should include: low vs. high, deflagrating vs. detonating, DOT shipping classifications, chemical classifications, etc. -

Potentially Explosive Chemicals*
Potentially Explosive Chemicals* Chemical Name CAS # Not 1,1’-Diazoaminonaphthalene Assigned 1,1-Dinitroethane 000600-40-8 1,2,4-Butanetriol trinitrate 006659-60-5 1,2-Diazidoethane 000629-13-0 1,3,5-trimethyl-2,4,6-trinitrobenzene 000602-96-0 1,3-Diazopropane 005239-06-5 Not 1,3-Dinitro-4,5-dinitrosobenzene Assigned Not 1,3-dinitro-5,5-dimethyl hydantoin Assigned Not 1,4-Dinitro-1,1,4,4-tetramethylolbutanetetranitrate Assigned Not 1,7-Octadiene-3,5-Diyne-1,8-Dimethoxy-9-Octadecynoic acid Assigned 1,8 –dihydroxy 2,4,5,7-tetranitroanthraquinone 000517-92-0 Not 1,9-Dinitroxy pentamethylene-2,4,6,8-tetramine Assigned 1-Bromo-3-nitrobenzene 000585-79-5 Not 2,2',4,4',6,6'-Hexanitro-3,3'-dihydroxyazobenzene Assigned 2,2-di-(4,4,-di-tert-butylperoxycyclohexyl)propane 001705-60-8 2,2-Dinitrostilbene 006275-02-1 2,3,4,6- tetranitrophenol 000641-16-7 Not 2,3,4,6-tetranitrophenyl methyl nitramine Assigned Not 2,3,4,6-tetranitrophenyl nitramine Assigned Not 2,3,5,6- tetranitroso nitrobenzene Assigned Not 2,3,5,6- tetranitroso-1,4-dinitrobenzene Assigned 2,4,6-Trinitro-1,3,5-triazo benzene 029306-57-8 Not 2,4,6-trinitro-1,3-diazabenzene Assigned Not 2,4,6-Trinitrophenyl trimethylol methyl nitramine trinitrate Assigned Not 2,4,6-Trinitroso-3-methyl nitraminoanisole Assigned 2,4-Dinitro-1,3,5-trimethyl-benzene 000608-50-4 2,4-Dinitrophenylhydrazine 000119-26-6 2,4-Dinitroresorcinol 000519-44-8 2,5-dimethyl-2,5-diydroperoxy hexane 2-Nitro-2-methylpropanol nitrate 024884-69-3 3,5-Dinitrosalicylic acid 000609-99-4 Not 3-Azido-1,2-propylene glycol dinitrate -

1. PUBLIC HEALTH STATEMENT This Statement Was Prepared to Give
TETRYL 1 1. PUBLIC HEALTH STATEMENT This Statement was prepared to give you information about tetryl and to emphasize the human health effects that may result from exposure to it. The Environmental Protection Agency (EPA) has identified 1,397 hazardous waste sites as the most serious in the nation. These sites make up the National Priorities List (NPL) and are the sites targeted for long-term federal clean-up activities. Tetryl has been found in at least 12 of the sites on the NPL. However, the number of NPL sites evaluated for tetryl is not known. As EPA evaluates more sites, the number of sites at which tetryl is found may increase. This information is important because exposure to tetryl may cause harmful health effects and because these sites are potential or actual sources of human exposure to tetryl. When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. You can be exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking substances containing the substance or by skin contact with it. If you are exposed to a substance such as tetryl, many factors will determine whether harmful health effects will occur and what the type and severity of those health effects will be. These factors include the dose (how much), the duration (how long), the route or pathway by which you are exposed (breathing, eating, drinking, or skin contact), the other chemicals to which you are exposed, and your individual characteristics such as age, gender, nutritional status, family traits, life-style, and state of health. -

Guide for the Selection of Commercial Explosives Detection Systems For
2.5.3.8 EXPRAY Field Test Kit EXPRAY is a unique, aerosol-based field test kit for the detection of what the manufacturer refers to as Group A explosives (TNT, DNT, picric acid, etc.), Group B explosives (Semtex H, RDX, PETN, NG, smokeless powder, etc.), and compounds that contain nitrates that are used in improvised explosives. Detection of explosive residue is made by observing a color change of the test paper. EXPRAY can be used in a variety of applications, and although in some aspects it does not perform as well as many of the other trace detectors discussed in this section, it costs only $250. This very low cost, coupled with simplicity and ease of use, may make it of interest to many law enforcement agencies (see the EXPRAY kit in fig. 13). The EXPRAY field kit2 is comprised of the following items: - one can of EXPRAY-1 for Group A explosives, - one can of EXPRAY-2 for Group B explosives, - one can of EXPRAY-3 for nitrate-based explosives (ANFO, black powder, and commercial and improvised explosives based on inorganic nitrates), - special test papers which prevent cross contamination. Figure 13. Photo of the EXPRAY Field Test Kit for explosives Initially, a suspected surface (of a package, a person’s clothing, etc.) is wiped with the special test paper. The paper is then sprayed with EXPRAY-1. The appearance of a dark violet-brown color indicates the presence of TNT, a blue-green color indicates the presence of DNT, and an orange color indicates the presence of other Group A explosives. -

Toxicological Profile for Tetryl
TOXICOLOGICAL PROFILE FOR TETRYL (2,4,6-Trinitrophenyl-N-methylnitramine) U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry June 1995 TETRYL ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. TETRYL iii UPDATE STATEMENT Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 TETRYL vii CONTRIBUTORS CHEMICAL MANAGERS (S)/AUTHOR (S): Carolyn Harper, Ph.D ATSDR, Division of Toxicology, Atlanta, GA Fernando Llados, Ph.D. Research Triangle Institute, Research Triangle Park, NC THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1. Green Border Review. Green Border review assures consistency with ATSDR policy. 2. Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying end points. 3. Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. 4. Quality Assurance Review. The Quality Assurance Branch assures that consistency across profiles is maintained, identifies any significant problems in format or content, and establishes that Guidance has been followed. TETRYL ix PEER REVIEW A peer review panel was assembled for tetryl. -

Quantifying the Stability of Trace Explosives Under Different
Talanta 165 (2017) 10–17 Contents lists available at ScienceDirect Talanta journal homepage: www.elsevier.com/locate/talanta ff Quantifying the stability of trace explosives under di erent environmental MARK conditions using electrospray ionization mass spectrometry☆ ⁎ Edward Siscoa, , Marcela Najarroa, Daniel Samarovb,Jeffrey Lawrencea a National Institute of Standards and Technology, Materials Measurement Science Division, Gaithersburg, MD, USA b National Institute of Standards and Technology, Statistical Engineering Division, Gaithersburg, MD, USA ARTICLE INFO ABSTRACT Keywords: This work investigates the stability of trace (tens of nanograms) deposits of six explosives: erythritol tetranitrate Trace explosives (ETN), pentaerythritol tetranitrate (PETN), cyclotrimethylenetrinitramine (RDX), cyclotetramethylenetetrani- Mass spectrometry tramine (HMX), 2,4,6-trinitrotoluene (TNT), and 2,4,6-trinitrophenylmethylnitramine (tetryl) to determine Degradation environmental stabilities and lifetimes of trace level materials. Explosives were inkjet printed directly onto Environmental stability substrates and exposed to one of seven environmental conditions (Laboratory, −4 °C, 30 °C, 47 °C, 90% relative humidity, UV light, and ozone) up to 42 days. Throughout the study, samples were extracted and quantified using electrospray ionization mass spectrometry (ESI-MS) to determine the stability of the explosive as a function of time and environmental exposure. Statistical models were then fit to the data and used for pairwise comparisons of the environments. Stability was found to be exposure and compound dependent with minimal sample losses observed for HMX, RDX, and PETN while substantial and rapid losses were observed in all conditions except −4 °C for ETN and TNT and in all conditions for tetryl. The results of this work highlight the potential fate of explosive traces when exposed to various environments. -

Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives Kelley L
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@Florida International University Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-7-2014 Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives Kelley L. Peters Florida International University, [email protected] Follow this and additional works at: http://digitalcommons.fiu.edu/etd Part of the Analytical Chemistry Commons, and the Other Chemistry Commons Recommended Citation Peters, Kelley L., "Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives" (2014). FIU Electronic Theses and Dissertations. Paper 1659. http://digitalcommons.fiu.edu/etd/1659 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida DEVELOPMENT OF PRESUMPTIVE AND CONFIRMATORY ANALYTICAL METHODS FOR THE SIMULTANEOUS DETECTION OF MULTIPLE IMPROVISED EXPLOSIVES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Kelley L. Peters 2014 To: Interim Dean Michael Heithaus College of Arts and Sciences This dissertation, written by Kelley L. Peters, and entitled Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives, having been approved in respect to style and intellectual content, is referred to you for judgment. We have read this dissertation and recommend that it be approved. -

Mine Disposal Handbook, Part 2, United States
PART II UNITED STATES UNDERWATER ORDNANCE CONFIDENTIAL TABLE OF CONTENTS PART I I - UNITED STATES UNDERWATER ORDNANCE CHAPTER 1 U. S. INFLUENCE MINES Page Mine Chart (Table I ) ............................................................................... 2 Introduction .............................................................................................. 3 kk 10-3 (10-6, 10-7, 10-8 k 10-9) ....................................................... 5 kk 1 2 - 0 ............................................................................................................... 9 Mk 12-1 ...................................................................................................... 11 Mk 12-3 k 12-4 ............................................................................................... 13 kk 13-0 (13-3 k 13-4) ...................................................................................... 13 kk 13-$ (13-6) ................................................................................................ 1$ Wk 1 6 -2 ........................................................................................................ 16c Mk 1 8 - 0 ........................................................................................................... 17 kk 2 1 - 0 ........................................................................................................ 19 kk 2 5 -0 ........................................................................................................ 21 kk 25-1 ................................................................................................... -

Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives Kelley L
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 11-7-2014 Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives Kelley L. Peters Florida International University, [email protected] DOI: 10.25148/etd.FI14110757 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Analytical Chemistry Commons, and the Other Chemistry Commons Recommended Citation Peters, Kelley L., "Development of Presumptive and Confirmatory Analytical Methods for the Simultaneous Detection of Multiple Improvised Explosives" (2014). FIU Electronic Theses and Dissertations. 1659. https://digitalcommons.fiu.edu/etd/1659 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida DEVELOPMENT OF PRESUMPTIVE AND CONFIRMATORY ANALYTICAL METHODS FOR THE SIMULTANEOUS DETECTION OF MULTIPLE IMPROVISED EXPLOSIVES A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in CHEMISTRY by Kelley L. Peters 2014 To: Interim Dean Michael Heithaus College of Arts and Sciences This dissertation, written by Kelley L. Peters, and entitled Development of Presumptive and Confirmatory Analytical Methods -

Sensitive Determination of Explosive Compounds in Water
APPLICATION NOTE 70745 Sensitive determination of explosive compounds in water Authors Goal Chen Jing, Xu Qun, To develop an efficient high-performance liquid chromatography (HPLC) Jeffrey Rohrer, method for the sensitive determination of 14 explosives and related Thermo Fisher Scientific, Shanghai, substances in tap water using on-line solid phase extraction (SPE) People’s Republic of China instead of the salting-out extraction specified in U.S. Environmental Protection Agency (EPA) Method 8330A Keywords Explosives, HPLC, Nitro Introduction Compounds, On-Line SPE, U.S. There has been increased attention on the persistence of residual EPA Method 8330A, Water Analysis explosive materials and their degradation products, which are substances that are highly toxic to the environment. Gas chromatography (GC) is one of the methods used to determine these substances, but because some explosive materials and their byproducts are thermally unstable or nonvolatile, this can result in inexact determinations. HPLC with UV detection is ideally suited for low-level determination because it is not subject to these limitations. 1 EPA Method 8330A describes an HPLC method with UV Previous methods have been used to complete the detection to determine 14 priority explosives and related separation and confirmation simultaneously on the substances (structures shown in Figure 1).1 Thermo Scientific™ UltiMate™ 3000 Dual Gradient Rapid Separation (RS) LC system.2 The sample This EPA method requires two columns for analysis. preparation procedure for low concentrations of the A C18 reversed-phase column is used as the primary target compounds in water, described in EPA Method column to separate the 14 compounds. However, not 8830A, is a salting-out extraction.