The Life of Ni 2
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Pennsylvania State University Center for Critical Minerals Final
The Pennsylvania State University Center for Critical Minerals Final Report on Cobalt Production in Pennsylvania: Context and Opportunities PREPARED FOR LEONARDO TECHNOLOGIES LLC., PREPARED BY Peter L. Rozelle Feifei Shi Mohammad Rezaee Sarma V. Pisupati Disclaimer This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. ii Executive Summary Cobalt is a critical mineral as defined by the U.S. Department of the Interior in response to Executive Order 13817 (2017). Cobalt metal is used in superalloys required in the hot gas path of stationary gas turbines and jet engines, as well as in samarium cobalt magnets, the domestic production capability of which was found to be essential to the National Defense under Presidential Determination 2019-20. Cobalt compounds are used in lithium-ion batteries, as are manganese compounds. The Defense Industrial Base Report, in response to Executive Order 13806 (2017), expressed concerns regarding the U.S. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Overview of the Nickel Market in Latin America and the Caribbean
INSG SECRETARIAT BRIEFING PAPER April 2021 – No.35 Overview of the Nickel market in Latin America and the Caribbean Ricardo Ferreira, Director of Market Research and Statistics Francisco Pinto, Manager of Statistical Analysis 1. Introduction At the suggestion of the Group’s Brazilian delegation, it was agreed by members that a report, based on INSG Insight No. 26, published in November 2015 entitled “An overview of the nickel industry in Latin America”, be prepared by the secretariat. This would include an update of the operations that resumed production (e.g. Falcondo in the Dominican Republic), and also discuss how the emerging battery market might influence nickel usage in the region as well as the possibility of nickel scrap usage. This detailed and comprehensive Insight report, the 35th in the series of INSG Insight briefing reports, provides members with the results of that research work. In Latin America and the Caribbean, the nickel producing countries are Brazil, Colombia, Cuba, Dominican Republic, Guatemala and Venezuela. The Dominican Republic stopped nickel mining and smelting in October 2013 but resumed production at the beginning of 2016. Venezuela has not produced since mid-2015. All of these countries mine nickel and process it further to produce intermediate or primary nickel – mainly ferronickel. Most of the mined ore is processed within each country, and then exported to overseas markets, but Brazil and Guatemala also export nickel ore. In terms of usage only Brazil and Mexico are relevant regarding the global nickel market. The first section of this report briefly describes existing nickel deposits in the region. -

Rebel Attacks on Nickel Ore Mines in the Surigao Del Norte Region
Mindanao, Philippines – rebel attacks on nickel ore mines in the Surigao del Norte region The attacks On 3 October 2011, three mining firms located in and around Claver town in the Surigao del Norte region of Mindanao, Philippines were attacked by the rebel group known as the New People’s Army (NPA). It was reported that the attacks were prompted by a refusal of the mining firms to pay a "revolutionary tax" demanded by the NPA. The affected mining firms are the Taganito Mining Corporation (TMC), Taganito HPAL Nickel Corporation and Platinum Group Metals Corporation (PGMC)1. According to news reports and Gard’s local correspondent in the Philippines, some 300 NPA rebels wearing police/military uniforms staged coordinated strikes on the three mining facilities. The rebels attacked TMC’s compound first, burning heavy equipment, service vehicles, barges and buildings. Other rebel groups simultaneously attacked the nearby PGCM and Taganito HPAL compounds. Unconfirmed reports indicate that, unfortunately, casualties were also experienced during the attacks. All foreign vessels anchored near Taganito at the time of the attacks left the anchorage safely. The current situation The Philippine Government has stated that the situation is now contained and has deployed additional troops in the area to improve security and pursue the NPA rebels. However, according to Gard’s local correspondent in the Philippines, threats of further attacks made by the NPA in a press release are believed by many to be real. According to the latest press releases (Reuters on 5 October), Nickel Asia Corporation has resumed operations at its biggest nickel mine TMC and expects to ship ore in the next three weeks. -
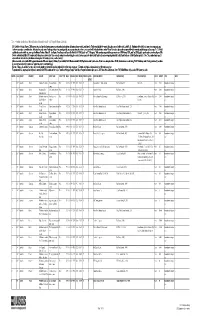
Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254)
Table1.—Attribute data for the map "Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254). [The United States Geological Survey (USGS) surveys international mineral industries to generate statistics on the global production, distribution, and resources of industrial minerals. This directory highlights the economically significant mineral facilities of Asia and the Pacific. Distribution of these facilities is shown on the accompanying map. Each record represents one commodity and one facility type for a single location. Facility types include mines, oil and gas fields, and processing plants such as refineries, smelters, and mills. Facility identification numbers (“Position”) are ordered alphabetically by country, followed by commodity, and then by capacity (descending). The “Year” field establishes the year for which the data were reported in Minerals Yearbook, Volume III – Area Reports: Mineral Industries of Asia and the Pacific. In the “DMS Latitiude” and “DMS Longitude” fields, coordinates are provided in degree-minute-second (DMS) format; “DD Latitude” and “DD Longitude” provide coordinates in decimal degrees (DD). Data were converted from DMS to DD. Coordinates reflect the most precise data available. Where necessary, coordinates are estimated using the nearest city or other administrative district.“Status” indicates the most recent operating status of the facility. Closed facilities are excluded from this report. In the “Notes” field, combined annual capacity represents the total of more facilities, plus additional -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -

The Nickel Silvers
Copper Development Association The Nickel Silvers Design Data and Applications 1965 Please note this publication is provided as an archive copy. The information given may therefore not be current. The Nickel Silvers Design Data and Applications 1965 Copper Development Association Copper Development Association is a non-trading organisation sponsored by the copper producers and fabricators to encourage the use of copper and copper alloys and to promote their correct and efficient application. Its services, which include the provision of technical advice and information, are available to those interested in the utilisation of copper in all its aspects. The Association also provides a link between research and user industries and maintains close contact with other copper development associations throughout the world. Website: www.copperinfo.co.uk Email: [email protected] Copyright: All information in this document is the copyright of Copper Development Association Disclaimer: Whilst this document has been prepared with care, Copper Development Association can give no warranty regarding the contents and shall not be liable for any direct, indirect or consequential loss arising out of its use Contents Contents ...............................................................................................................................................................1 Introduction .........................................................................................................................................................2 -

Special-Purpose Nickel Alloys
© 2000 ASM International. All Rights Reserved. www.asminternational.org ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys (#06178G) Special-Purpose Nickel Alloys NICKEL-BASE ALLOYS have a number of meet special needs. The grades considered in ganese, and copper, a 0.005% limit on iron, and unique properties, or combinations of proper- this section include the following: a 0.02% limit on carbon. This high purity re- ties, that allow them to be used in a variety of sults in lower coefficient of expansion, electri- specialized applications. For example, the high • Nickel 200 (99.6% Ni, 0.04% C) cal resistivity, Curie temperature, and greater resistivity (resistance to flow of electricity) and • Nickel 201 (99.6% Ni, 0.02% C maximum) ductility than those of other grades of nickel heat resistance of nickel-chromium alloys lead • Nickel 205 (99.6% Ni, 0.04% C, 0.04% Mg) and makes Nickel 270 especially useful for to their use as electric resistance heating ele- • Nickel 233 (see composition in table that fol- some electronics applications such as compo- ments. The soft magnetic properties of lows) nents of hydrogen thyratrons and as a substrate nickel-iron alloys are employed in electronic • Nickel 270 (99.97% Ni) for precious metal cladding. devices and for electromagnetic shielding of computers and communication equipment. Iron- Composition limits and property data on sev- eral of these grades can be found in the article nickel alloys have low expansion characteris- Resistance Heating Alloys tics as a result of a balance between thermal ex- “Wrought Corrosion-Resistant Nickels and pansion and magnetostrictive changes with Nickel Alloys” in this Handbook. -

Properties of Gold-Nickel Alloy Brazed Joints in High Temperature Materials
Properties of Gold-Nickel Alloy Brazed Joints in High Temperature Materials Professor Jakob Colbus University of Saarland, Saarbrücken, West Germany and Karl Friedrich Zimmermann Degussa, Wolfgang, West Germany The usefulness of gold-nickel alloys as brazing media in the aero- engine, electronic, nuclear power and spacecraft industries is illustrated by data on the corrosion and oxidation resistance of joints made with these alloys in various heat-resistant materials and on their resistance to fracture under the influence of static, dynamic and impact loads in tests at subzero, normal and elevated temperatures. Gold solders have been in use for many years, Brazing temperature in the range 900 to 1000°C. especially in the jewellery trade and in dentistry. Ability to make joints that retain their strength The solders used in the fabrication of jewellery and ductility at elevated temperatures. contain silver, copper, cadmium, and zinc, which are Good resistance to oxidation and corrosion. added to ensure that the colour and the gold content All these requirements can be satisfied by several of a given solder matches the corresponding charac- alloys in the gold-nickel system, whose constitutional teristics of the soldered article. The solders used in diagram is shown in Figure 1. The 18 per cent dental practice often contain other precious metals nickel-gold alloy is the most suitable one to serve as a such as iridium, palladium, and platinum introduced brazing medium. Its melting point of 950°C is the to increase their corrosion resistance, the most important property of solders of this kind. In both these applications the soldering is done in air with WEIGHT PERCENT NICKEL the aid of a gas torch and a suitable flux. -

Mechanical Properties of Electroformed Nickel Cobalt Alloys
Mechanical Properties of Electroformed Nickel Cobalt Alloys B. Stein, P. Jaeger and C. Przybyla, NiCoForm, Inc., Rochester, New York, USA Abstract Hardness was measured on ½" - thick stainless steel blocks plated with at least 0.02" of Ni-Co on a Mechanical properties of nickel-cobalt alloys (0-10% Rockwell superficial hardness tester (Wilson Co) electroformed with low internal stress in Instruments Mod. 3 JS) with a 15kg load. The sulfamate-based solutions were investigated in as average of three readings was taken for each data point deposited and heat treated conditions. It was in tensile and hardness tests. demonstrated that alloy properties can be modified by selecting deposition conditions and alloy composition. Both foil samples and hardness test blocks were Recommendations on alloy selection are made based electroformed at 1-3 A/Dm2 in two proprietary Ni-Co on application requirements. alloy electroforming chemistries, one designed for enhanced deposit hardness, the other - for high tensile The higher strength and hardness of nickel-cobalt alloy strength. Deposit internal stress was measured directly electrodeposits compared to nickel stimulates their in the plating tanks as described elsewhere [1], while increasing use in electroforming and as engineering tensile and hardness samples were being coatings. Many advanced electroformed products such electroformed. Tensile and hardness tests were as bellows, contacts, optical and nanofluidic molds can performed on samples in the as plated and heat treated only be produced consistently if the deposits’ (2 hours at the set temperature) conditions. properties are well understood and properly controlled Representative foil samples were chemically analyzed by the process engineer. -

Mt Keith Presentation
Australian Site Tour Mt Keith Operation Jaco Harwig General Manager 28 October 2008 Important notices Reliance on third party information The views expressed here contain information that have been derived from publicly available sources that have not been independently verified. No representation or warranty is made as to the accuracy, completeness or reliability of the information. This presentation should not be relied upon as a recommendation or forecast by BHP Billiton. Forward looking statements This presentation includes forward-looking statements within the meaning of the U.S. Securities Litigation Reform Act of 1995 regarding future events and the future financial performance of BHP Billiton. These forward-looking statements are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results to differ materially from those expressed in the statements contained in this presentation. For more detail on those risks, you should refer to the sections of our annual report on Form 20- F for the year ended 30 June 2008 entitled “Risk factors” , “Forward looking statements” and “Operating and financial review and prospects” filed with the U.S. Securities and Exchange Commission. No offer of securities Nothing in this release should be construed as either an offer to sell or a solicitation of an offer to buy or sell BHP Billiton securities in any jurisdiction. Cautionary Note to US Investors The SEC generally permits mining companies in their filings with the SEC to disclose only those mineral deposits that the company can economically and legally extract.