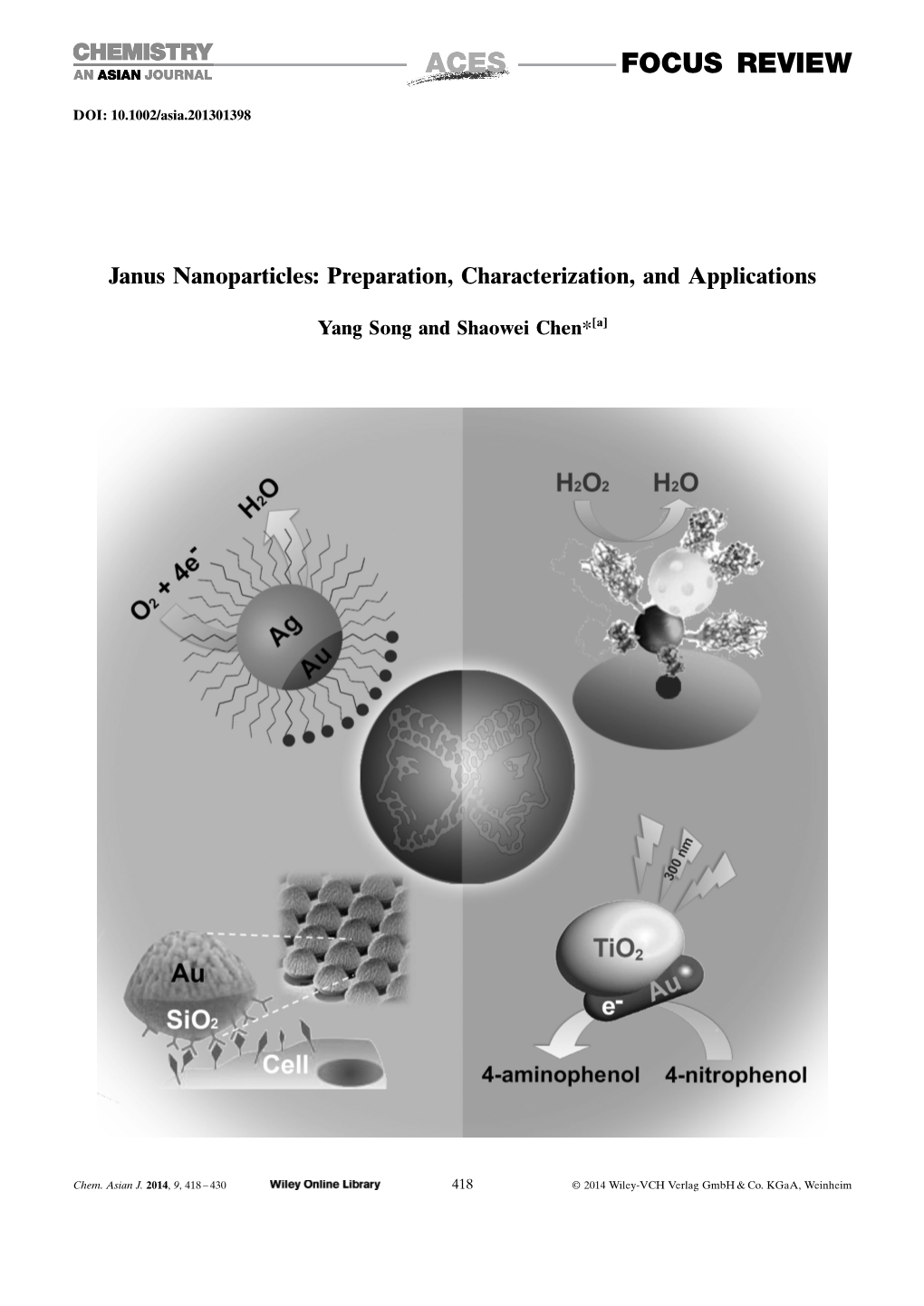
Janus Nanoparticles: Preparation, Characterization, and Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Substrate Wettability Guided Oriented Self Assembly of Janus Particles
www.nature.com/scientificreports OPEN Substrate wettability guided oriented self assembly of Janus particles Meneka Banik1, Shaili Sett2, Chirodeep Bakli3, Arup Kumar Raychaudhuri2, Suman Chakraborty4* & Rabibrata Mukherjee1* Self-assembly of Janus particles with spatial inhomogeneous properties is of fundamental importance in diverse areas of sciences and has been extensively observed as a favorably functionalized fuidic interface or in a dilute solution. Interestingly, the unique and non-trivial role of surface wettability on oriented self-assembly of Janus particles has remained largely unexplored. Here, the exclusive role of substrate wettability in directing the orientation of amphiphilic metal-polymer Bifacial spherical Janus particles, obtained by topo-selective metal deposition on colloidal Polymestyere (PS) particles, is explored by drop casting a dilute dispersion of the Janus colloids. While all particles orient with their polymeric (hydrophobic) and metallic (hydrophilic) sides facing upwards on hydrophilic and hydrophobic substrates respectively, they exhibit random orientation on a neutral substrate. The substrate wettability guided orientation of the Janus particles is captured using molecular dynamic simulation, which highlights that the arrangement of water molecules and their local densities near the substrate guide the specifc orientation. Finally, it is shown that by spin coating it becomes possible to create a hexagonal close-packed array of the Janus colloids with specifc orientation on diferential wettability substrates. -

Adhesion Force Studies of Janus Nanoparticles
8544 Langmuir 2007, 23, 8544-8548 Adhesion Force Studies of Janus Nanoparticles Li-Ping Xu, Sulolit Pradhan, and Shaowei Chen* Department of Chemistry and Biochemistry, UniVersity of California, 1156 High Street, Santa Cruz, California 95064 ReceiVed March 15, 2007. In Final Form: May 16, 2007 Janus nanoparticles represent a unique nanoscale analogue to the conventional surfactant molecules, exhibiting hydrophobic characters on one side and hydrophilic characters on the other. Yet, direct visualization of the asymmetric surface structures of the particles remains a challenge. In this paper, we used a simple technique based on AFM adhesion force measurements to examine the two distinctly different hemispheres of the Janus particles at the molecular level. Experimentally, the Janus nanoparticles were prepared by ligand exchange reactions at the air-water interface. The particles were then immobilized onto a substrate surface with the particle orientation controlled by the chemical functionalization of the substrate surface, and an AFM adhesion force was employed to measure the interactions between the tip of a bare silicon probe and the Janus nanoparticles. It was found that when the hydrophilic side of the particles was exposed, the adhesion force was substantially greater than that with the hydrophobic side exposed, as the silicon probes typically exhibit hydrophilic properties. These studies provide further confirmation of the amphiphilic nature of the Janus nanoparticles. Introduction both electrical and color anisotropy, they may be used in electronic paper.19,20 Janus particles coated with different chemical groups Nanoparticles have long been fascinating objects due to their can also be derivatized into bifunctional carriers useful for potential applications as novel building blocks for the fabrication catalysis, sensing, drug delivery, etc. -

Molecular Insights of Adsorption and Structure of Surfactants and Inorganic Ions at Fluid – Liquid Interfaces
Molecular Insights of Adsorption and Structure of Surfactants and Inorganic Ions at Fluid – Liquid Interfaces Afshin Asadzadeh Shahir B.Sc. Applied Chemistry, M.Sc. Physical Chemistry A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2017 School of Chemical Engineering Abstract Traditional approaches to studying fluid – liquid interfaces include the macroscopic measuring of interfacial properties such as surface tension and matching the collected data against adsorption models. This method is capable of producing valuable data about the thermodynamics of adsorption and has been widely used by the community to extract information about the adsorption of thousands of different surface-active molecules. Nonetheless, this methodology cannot produce any molecular-level information about the microscopic structure of adsorption layers and interfaces. As a result, the molecular origins of many interfacial phenomena remained unknown until the advent of surface-sensitive techniques such as computer simulation and non-linear spectroscopy. The new insights provided by these methods have challenged the traditional views about the origins of some interfacial phenomena and promise modification of classical theories which were developed to explain these phenomena. In general, this thesis aims to study the interfacial structure and adsorption of ionic surfactants, some surface-active alcohols as model nonionic surfactants including, n-pentanol, methyl isobutyl carbinol (MIBC) and n-hexanol and inorganic salts including LiCl, NaCl and CsCl at both microscopic and macroscopic levels. The employed methodology involves a combination of traditional adsorption modelling with some macroscopic measurements and sum frequency generation (SFG) spectroscopy, which is capable of distinguishing between bulk and interfacial molecules. -

Large Amphiphilic Janus Microgels As Droplet Stabilizers Bobby Haney, Jörg G
Subscriber access provided by Harvard Library Surfaces, Interfaces, and Applications Absorbent-Adsorbates: Large Amphiphilic Janus Microgels as Droplet Stabilizers Bobby Haney, Jörg G. Werner, David A. Weitz, and Subramanian Ramakrishnan ACS Appl. Mater. Interfaces, Just Accepted Manuscript • DOI: 10.1021/acsami.0c11408 • Publication Date (Web): 29 Jun 2020 Downloaded from pubs.acs.org on July 10, 2020 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts. is published by the American Chemical Society. -

Synthesis of Polystyrene–Polyphenylsiloxane Janus Particles Through Colloidal Assembly with Unexpected High Selectivity
polymers Article Synthesis of Polystyrene–Polyphenylsiloxane Janus Particles through Colloidal Assembly with Unexpected High Selectivity: Mechanistic Insights and Their Application in the Design of Polystyrene Particles with Multiple Polyphenylsiloxane Patches Daniel Mann 1,2, Stefanie Voogt 1,2,3, Helmut Keul 1,2, Martin Möller 1,2, Marcel Verheijen 4,5 and Pascal Buskens 1,2,3,6,* 1 DWI—Leibniz Institute for Interactive Materials e.V., Forckenbeckstr. 50, 52056 Aachen, Germany; [email protected] (D.M.); [email protected] (S.V.); [email protected] (H.K.); [email protected] (M.M.) 2 Institute for Technical and Macromolecular Chemistry, RWTH Aachen University, Worringerweg 2, 52074 Aachen, Germany 3 Zuyd University of Applied Sciences, Nieuw Eyckholt 300, Postbus 550, 6400 AN Heerlen, The Netherlands 4 Philips Innovation Labs, High Tech Campus 11, 5656 AE Eindhoven, The Netherlands; [email protected] 5 Department of Applied Physics, Eindhoven University of Technology, P.O. Box 513, 5600 MB Eindhoven, The Netherlands 6 The Netherlands Organisation for Applied Scientific Research (TNO), De Rondom 1, 5612 AP Eindhoven, The Netherlands * Correspondence: [email protected]; Tel.: +31-88-866-2990 Received: 1 September 2017; Accepted: 26 September 2017; Published: 28 September 2017 Abstract: Janus particles are of great research interest because of their reduced symmetry, which provides them with unique physical and chemical properties. Such particles can be prepared from spherical structures through colloidal assembly. Whilst colloidal assembly has the potential to be a low cost and scalable process, it typically lacks selectivity. As a consequence, it results in a complex mixture of particles of different architectures, which is tedious to purify. -

Janus Particles for (Bio)Sensing
View metadata, citation and similarbrought COREpapers to youat core.ac.ukby provided by EPrints Complutense Dedicated to our dear friend Dr. Joseph Wang for his 70th birthday, a scientist unrepeatable and still better person Janus particles for (bio)sensing P. Yánez-Sedeñoa, S. Campuzanoa,*, J.M. Pingarróna,b,* a Departamento de Química Analítica, Facultad de Ciencias Químicas, Universidad Complutense de Madrid. Avda. Complutense s/n, E-28040 Madrid, Spain. Fax: b IMDEA Nanoscience, Ciudad Universitaria de Cantoblanco, 28049 Madrid, Spain E-mails: [email protected]; [email protected]; [email protected]. Tel. 34913944315, fax 34 913944329. * to whom correspondence should be addressed 1 Abstract This review article sheds useful insight in the use of Janus nanoparticles for (bio)sensing in connection with optical and electrochemical transduction. After a brief introduction of the main properties, types and fabrication strategies of Janus nanoparticles, selected applications for their use in electrochemical and optical biosensing are critically discussed. Highlighted examples illustrate the great versatility and interesting possibilities offered by these smart multifunctional nanoparticles for (bio)sensing of relevant analytes operating both in static and dynamic modes. Progress made so far demonstrate their suitability for designing single- or multiplexed (bio)sensing strategies for target analytes of different nature (organic and inorganic compounds, proteins, cells and oligomers) with relevance in clinical (H2O2, glucose, cholesterol, CEA, human IgG, propranolol, bacterial and tumor cells) and environmental (lead and organophosphorous nerve agents) fields. Key future challenges and envisioned opportunities of the use of Janus nanoparticles in the (bio)sensing field are also discussed. Keywords: Janus particles; Janus micromotors; biosensing; optical; electrochemical. -

Adsorption at the Liquid/Gas Interface
1.1. SurfaceSurface tensiontension ofof solutionssolutions Adsorption at the liquid/gas interface In the case of solutions, contrary to pure liquids, simultaneously with the changes of surface area, the surface tension γ may change. If a two-component solution behaves as a regular one, its surface tension changes as a function of the surface composition, according to the equation derived by Prigogine and Defay. γ = γ1x1 + γ2x2 −βpx1x2 (1) where: γγγ1 and γγγ2 are the surface tensions of pure liquid 1 and 2, respectively, x1 and x2 are the molar rations of these liquids, βp is the semi-empirical constant. Just to recall, a regular solution is a solution that diverges from the behavior of an ideal one only moderately. For regular solutions: o o o o Cpi −Cpi =0 µi −µi =RT ln ai Si − Si =−RT ln xi Hi −Hi =f (xi ) Note that the Margules function always contains the opposite mole fraction. In contrast to the case of ideal solutions, regular solutions do possess an enthalpy of mixing and their volume solutions are not strictly additive and must be calculated from the partial molar volumes that are a function of x. Adsorption at the liquid/gas interface The activity coefficients of the liquids (expressed via molar ratio) which form the mixed solutions satisfy the following relations (Margules functions): 2 RT ln f1 = − αx 2 (2) and 2 RT ln f 2 = −αx1 (3) A typical mixed solution of two liquids is acetone-chloroform , whose surface tension is shown in Fig.1.1. The surface tensions of these liquids are comparable, ( γ = 23.7 mN m –1 for acetone , and γ = 27.1 mN m –1 for chloroform ). -

Phospholipid Monolayers Supported on Spun Cast Polystyrene Films John T
Langmuir 2003, 19, 2275-2283 2275 Phospholipid Monolayers Supported on Spun Cast Polystyrene Films John T. Elliott,*,†,‡ Daniel L. Burden,§ John T. Woodward,† Amit Sehgal,| and Jack F. Douglas| Biotechnology Division, Chemical Science and Technology Laboratory, and Polymers Division, Materials Science and Engineering Laboratory, National Institute of Standards and Technology, 100 Bureau Drive, Gaithersburg, Maryland 20899, and Chemistry Department, Wheaton College, Wheaton, Illinois 60817 Received June 12, 2002. In Final Form: November 19, 2002 We investigated the use of spun cast polystyrene (PS) films as a hydrophobic solid support for phospholipid monolayers. Fluorescent microscopy indicated that a 1-palmitoyl-2-oleoyl-SN-glycero-3-phosphocholine (POPC) monolayer containing fluorescent lipid formed on ∼80 nm thick films of 2450 Mr PS exposed to phospholipid vesicle solutions. Qualitative fluorescence photobleaching experiments suggested that the phospholipid layer was continuous and the lipid diffusion rate was similar to those observed in glass- supported POPC bilayers. Examination of lipid diffusion by real-time, single-molecule fluorescence detection and fluorescence correlation spectroscopy (FCS) indicated that the lipid diffusion contained multiple components. We interpreted this as being caused by the presence of surface heterogeneities that confine lipid diffusion. In situ atomic force microscopy revealed the presence of 5-20 nm outgrowths on the PS film resulting from surface rearrangement upon exposure of the film to aqueous conditions. It is possible that such rearrangements play a role in determining the lipid translational dynamics. Our studies indicate that PS films can be suitable supports for phospholipid monolayers, but that immersion into an aqueous environment can significantly influence the film topography and the dynamics of the lipid in the supported monolayer. -

Microemulsion Microstructure(S): a Tutorial Review
nanomaterials Review Microemulsion Microstructure(s): A Tutorial Review Giuseppe Tartaro 1 , Helena Mateos 1 , Davide Schirone 1 , Ruggero Angelico 2 and Gerardo Palazzo 1,* 1 Department of Chemistry, and CSGI (Center for Colloid and Surface Science), University of Bari, via Orabona 4, 70125 Bari, Italy; [email protected] (G.T.); [email protected] (H.M.); [email protected] (D.S.) 2 Department of Agricultural, Environmental and Food Sciences (DIAAA), University of Molise, I-86100 Campobasso, Italy; [email protected] * Correspondence: [email protected] Received: 30 June 2020; Accepted: 18 August 2020; Published: 24 August 2020 Abstract: Microemulsions are thermodynamically stable, transparent, isotropic single-phase mixtures of two immiscible liquids stabilized by surfactants (and possibly other compounds). The assortment of very different microstructures behind such a univocal macroscopic definition is presented together with the experimental approaches to their determination. This tutorial review includes a necessary overview of the microemulsion phase behavior including the effect of temperature and salinity and of the features of living polymerlike micelles and living networks. Once these key learning points have been acquired, the different theoretical models proposed to rationalize the microemulsion microstructures are reviewed. The focus is on the use of these models as a rationale for the formulation of microemulsions with suitable features. Finally, current achievements and challenges of the use of microemulsions are reviewed. Keywords: microemulsions; wormlike micelles; packing parameter; flexible surface model; hydrophilic–lipophilic difference (HLD); net average curvature (NAC) 1. Introduction As a matter of fact, water and oil do not mix. There are several applications in which this is a bitter truth and the coexistence of oil and water as separate macroscopic phases, interacting just through a small interface, is highly disappointing. -

Solid Supported Lipid Monolayer: from Biophysical Properties to Sensor Application
Aix-Marseille Université Thèse de Doctorat Pour l’obtention du grade de Docteur de l’université d’Aix Marseille Discipline : Physique Ecole doctorale Physique et Sciences de la Matière Présentée par Racha EL ZEIN Solid supported lipid monolayer: From biophysical properties to sensor application Centre Interdisiplinaire de Nano-sciences à Marseille (CINaM)-CNRS Campus de Luminy, Case 913, 13288 Marseille Cedex 9. Soutenance le : 15 mai 2013. Rapporteurs: Dominique Ausserré, IMMM, Université du Maine Thierry Charitat, ICS, Université de Strasbourg Examinateurs: Catherine Henry de Villeneuve, LPMC, Ecole Polytechnique Pierre-Henri Puech, LAI, Aix-Marseille Université Hervé Dallaporta, CINaM, Aix-Marseille Université (Directeur de thèse) Anne Charrier, CINaM, Aix-Marseille Université (Directrice de thèse) 2 Table de matière Introduction .............................................................................................................................. 9 1 Experimental techniques ................................................................................................. 15 1.1 Atomic Force Microscopy (AFM) .............................................................................. 15 1.1.1 Introduction .................................................................................................... 15 1.1.2 Description ...................................................................................................... 15 1.1.3 Forces in AFM ................................................................................................. -

Orientational Texture of Lipid Bilayer and Monolayer Domains
Orientational Texture of Lipid Bilayer and Monolayer Domains PhD Thesis Jes Dreier Supervised by Associate Professor Adam Cohen Simonsen MEMPHYS – center for biomembrane physics Department of1 Physics, Chemistry and Pharmacy MEMPHYS University of Southern Denmark ovember 2012 Preface his thesis covers the scientific work I have conducted during the last three year during my PhD education in biophysic at MEMPHYS – center for T biomembrane physics, Institute of Physics, Chemistry and Pharmacy, University of Southern Denmark. This work has been done under the qualified supervision of Associated Professor Adam Cohen Simonsen, and I am thankful for his help and advice during the last three years. Research Assistant Professor Jonathan R. Brewer should be acknowledged for the valuable help regarding the 2-photon microscope, its use, and interpretation of the results. I am very grateful to have had the opportunity to spend 6 month at University California Davis during my PhD. I owe many thanks to Professor Tonya Kuhl and Professor Margie Longo and their respectively research groups, for the help during my time there. I would like to acknowledge all the people at MEMPHYS for the help, support, and for making MEMPHYS what it is. A special thanks goes to the Ph.D. students Morten Christensen, Thomas E. Rasmussen and Mathias P. Clausen, both the help with this thesis, but also for countless discussions, (and much need coffee breaks) during my time at MEMPHYS. Your help have been invaluable. Most importantly a special thanks to my wife Signe, especially for the incredible support during the writing process of this thesis. The work in this thesis has lead to the following papers and manuscripts: J. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0037234 A1 PRUDHOMME Et Al
US 20170037234A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0037234 A1 PRUDHOMME et al. (43) Pub. Date: Feb. 9, 2017 (54) POLYMERNANOPARTICLES Publication Classification (71) Applicant: THE TRUSTEES OF PRINCETON (51) Int. Cl. UNIVERSITY, Princeton, NJ (US) COSL 25/06 (2006.01) A6II 47/32 (2006.01) (72) Inventors: Robert K. PRUDHOMME, BOI 3L/26 (2006.01) Lawrenceville, NJ (US); Rodney D. COSL 4700 (2006.01) PRIESTLEY, Princeton, NJ (US); Rui BOI 3L/06 (2006.01) LIU, Princeton, NJ (US); Chris SOSA, BOI 3L/28 (2006.01) Princeton, NJ (US) A6IR 9/16 (2006.01) (73) Assignee: THE TRUSTEES OF PRINCETON AOIN 25/10 (2006.01) UNIVERSITY, Princeton, NJ (US) (52) U.S. Cl. (21) Appl. No.: 15/121,715 CPC .................. C08L 25/06 (2013.01); A61K 9/16 (2013.01); A61K 47/32 (2013.01); A0IN 25/10 (22) PCT Fed: Feb. 25, 2015 (2013.01); C08L 47/00 (2013.01); B01J 31/06 (2013.01); B01J 3 I/28 (2013.01); B01J 31/26 (86) PCT No.: PCT/US 15/17590 (2013.01); B01.J 223 1/641 (2013.01) S 371 (c)(1), (2) Date: Aug. 25, 2016 Related U.S. Application Data (57) ABSTRACT (60) Provisional application No. 61/944,784, filed on Feb. 26, 2014, provisional application No. 62/042,515, Polymer nanoparticles, including Janus nanoparticles, and filed on Aug. 27, 2014. methods of making them are described. Patent Application Publication Feb. 9, 2017. Sheet 1 of 5 US 2017/0037234 A1 5 Patent Application Publication Feb. 9, 2017. Sheet 2 of 5 US 2017/0037234 A1 s -- PSIPRATIO (st8) FG.