(RTK) Ephb1, Ephb2 and Ephb4 in Colorectal Cancer
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
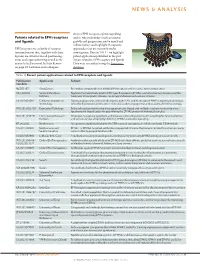
Patents Related to EPH Receptors and Ligands
NEWS & ANALYSIS discuss EPH receptor–ephrin signalling Patents related to EPH receptors and its role in disorders such as tumour and ligands growth and progression, nerve injury and inflammation, and highlight therapeutic EPH receptors are a family of receptor approaches that are currently under tyrosine kinases that, together with their investigation. Here in TABLE 1 we highlight ligands, are involved in cell positioning, patent applications published in the past tissue and organ patterning as well as the 3 years related to EPH receptors and ligands. control of cell survival. In their Review Data were researched using the Espacenet on page 39, Lackman and colleagues database. Table 1 | Recent patent applications related to EPH receptors and ligands Nature Reviews | Drug Discovery Publication Applicants Subject numbers NZ 581397 AstraZeneca Pyrimidine compounds that inhibit EPH receptors and are useful for treating cancer HK 1108702 Sanford-Burnham Peptides that selectively bind to EPH type-B receptors (EPHBs); useful for tumour imaging and the Institute treatment of neoplastic disease, neurological disease and vascular disease US 2013091591 California Institute of During angiogenesis, arterial cells express ephrin B2, and its receptor EPHB4 is expressed on venous Technology cells; this distinction can be used in methods to alter angiogenesis and to assess the effect of drugs WO 2013052710 Expression Pathology Selected reaction monitoring mass spectrometry-based and multiple reaction monitoring mass spectrometry-based assays for quantifying -

Ephb3 Suppresses Non-Small-Cell Lung Cancer Metastasis Via a PP2A/RACK1/Akt Signalling Complex
ARTICLE Received 7 Nov 2011 | Accepted 11 Jan 2012 | Published 7 Feb 2012 DOI: 10.1038/ncomms1675 EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex Guo Li1, Xiao-Dan Ji1, Hong Gao1, Jiang-Sha Zhao1, Jun-Feng Xu1, Zhi-Jian Sun1, Yue-Zhen Deng1, Shuo Shi1, Yu-Xiong Feng1, Yin-Qiu Zhu1, Tao Wang2, Jing-Jing Li1 & Dong Xie1 Eph receptors are implicated in regulating the malignant progression of cancer. Here we find that despite overexpression of EphB3 in human non-small-cell lung cancer, as reported previously, the expression of its cognate ligands, either ephrin-B1 or ephrin-B2, is significantly downregulated, leading to reduced tyrosine phosphorylation of EphB3. Forced activation of EphB3 kinase in EphB3-overexpressing non-small-cell lung cancer cells inhibits cell migratory capability in vitro as well as metastatic seeding in vivo. Furthermore, we identify a novel EphB3-binding protein, the receptor for activated C-kinase 1, which mediates the assembly of a ternary signal complex comprising protein phosphatase 2A, Akt and itself in response to EphB3 activation, leading to reduced Akt phosphorylation and subsequent inhibition of cell migration. Our study reveals a novel tumour-suppressive signalling pathway associated with kinase-activated EphB3 in non-small-cell lung cancer, and provides a potential therapeutic strategy by activating EphB3 signalling, thus inhibiting tumour metastasis. 1 Key Laboratory of Nutrition and Metabolism, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and Graduate School of Chinese Academy of Sciences, Shanghai 200031, China. 2 The Eastern Hepatobiliary Surgery Hospital, the Second Military Medical University, Shanghai 200433, China. -

Anti-Human EPHA4 / EPH Receptor A4 (Internal) Polyclonal Antibody
For Research Use Only. REV: 9/9/2014 Anti-Human EPHA4 / EPH Receptor A4 (Internal) Polyclonal Antibody CatalogID MC-2841 Target Protein EPH receptor A4 (EPHA4) Synonyms EPHA4 Antibody, CEK8 Antibody, EK8 Antibody, Ephrin type-A receptor 4 Antibody, HEK8 Antibody, SEK Antibody, TYRO1 Antibody, TYRO1 protein tyrosine kinase Antibody, Tyrosine-protein kinase TYRO1 Antibody, EPH receptor A4 Antibody, EPH-like kinase 8 Antibody Family / Subfamily Protein Kinase / Ephrin Receptor Host EPHA4 antibody was produced in Rabbit Clonality Polyclonal Immunogen Species EPHA4 / EPH Receptor A4 antibody was raised against Human Antigen Type Synthetic peptide Immunogen EPHA4 / EPH Receptor A4 antibody was raised against synthetic 18 amino acid peptide from internal region of human EPHA4. Percent identity with other species by BLAST analysis: Human, Gorilla, Gibbon, Monkey, Marmoset, Panda, Bovine, Dog, Bat, Horse, Pig (100%); Mouse, Rat, Hamster, Elephant, Rabbit, Opossum, Platypus, Xenopus (94%); Turkey, Chicken (89%); Lizard (83%). Specificity Human EPHA4. BLAST analysis of the peptide immunogen showed no homology with other human proteins. Epitope Internal Reactivity Human (human tissues have been tested in IHC with FFPE) Predicted Reactivity Gorilla, Gibbon, Monkey, Bat, Bovine, Dog, Horse, Pig, Mouse, Rat, Hamster, Rabbit, Xenopus Purification Immunoaffinity purified Presentation PBS, 0.1% sodium azide. Recommended Storage Long term: -70°C; Short term: +4°C Uses IHC - Paraffin (3 μg/ml) (Optimal dilution to be determined by the researcher) Size 50 µg Concentration 1 mg/ml For research use only. Not intended for human, diagnostic, therapeutic, or drug use. Immunohistochemistry Anti-EPHA4 antibody MC-2841 IHC of human kidney, glomerulus. Immunohistochemistry of formalin-fixed, paraffin- embedded tissue after heat-induced antigen retrieval. -

Structural and Functional Insights Into the Transmembrane Domain Association of Eph Receptors
International Journal of Molecular Sciences Review Structural and Functional Insights into the Transmembrane Domain Association of Eph Receptors Amita R. Sahoo 1 and Matthias Buck 1,2,3,4,* 1 Department of Physiology and Biophysics, School of Medicine, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA; [email protected] 2 Department of Neurosciences, School of Medicine, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA 3 Department of Pharmacology, School of Medicine, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA 4 Case Comprehensive Cancer Center, School of Medicine, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA * Correspondence: [email protected] Abstract: Eph receptors are the largest family of receptor tyrosine kinases and by interactions with ephrin ligands mediate a myriad of processes from embryonic development to adult tissue homeostasis. The interaction of Eph receptors, especially at their transmembrane (TM) domains is key to understanding their mechanism of signal transduction across cellular membranes. We review the structural and functional aspects of EphA1/A2 association and the techniques used to investigate their TM domains: NMR, molecular modelling/dynamics simulations and fluorescence. We also introduce transmembrane peptides, which can be used to alter Eph receptor signaling and we provide a perspective for future studies. Citation: Sahoo, A.R.; Buck, M. Keywords: receptor tyrosine kinase (RTKs); Eph receptors; TM dimerization; transmembrane Structural and Functional Insights domain (TMD) into the Transmembrane Domain Association of Eph Receptors. Int. J. Mol. Sci. 2021, 22, 8593. https:// doi.org/10.3390/ijms22168593 1. The Family of Eph Receptors, Their Domain Structure and Function Erythropoietin-producing hepatocellular carcinoma receptors (Ephs) represent the Academic Editors: Dimitar B. -
HCC and Cancer Mutated Genes Summarized in the Literature Gene Symbol Gene Name References*
HCC and cancer mutated genes summarized in the literature Gene symbol Gene name References* A2M Alpha-2-macroglobulin (4) ABL1 c-abl oncogene 1, receptor tyrosine kinase (4,5,22) ACBD7 Acyl-Coenzyme A binding domain containing 7 (23) ACTL6A Actin-like 6A (4,5) ACTL6B Actin-like 6B (4) ACVR1B Activin A receptor, type IB (21,22) ACVR2A Activin A receptor, type IIA (4,21) ADAM10 ADAM metallopeptidase domain 10 (5) ADAMTS9 ADAM metallopeptidase with thrombospondin type 1 motif, 9 (4) ADCY2 Adenylate cyclase 2 (brain) (26) AJUBA Ajuba LIM protein (21) AKAP9 A kinase (PRKA) anchor protein (yotiao) 9 (4) Akt AKT serine/threonine kinase (28) AKT1 v-akt murine thymoma viral oncogene homolog 1 (5,21,22) AKT2 v-akt murine thymoma viral oncogene homolog 2 (4) ALB Albumin (4) ALK Anaplastic lymphoma receptor tyrosine kinase (22) AMPH Amphiphysin (24) ANK3 Ankyrin 3, node of Ranvier (ankyrin G) (4) ANKRD12 Ankyrin repeat domain 12 (4) ANO1 Anoctamin 1, calcium activated chloride channel (4) APC Adenomatous polyposis coli (4,5,21,22,25,28) APOB Apolipoprotein B [including Ag(x) antigen] (4) AR Androgen receptor (5,21-23) ARAP1 ArfGAP with RhoGAP domain, ankyrin repeat and PH domain 1 (4) ARHGAP35 Rho GTPase activating protein 35 (21) ARID1A AT rich interactive domain 1A (SWI-like) (4,5,21,22,24,25,27,28) ARID1B AT rich interactive domain 1B (SWI1-like) (4,5,22) ARID2 AT rich interactive domain 2 (ARID, RFX-like) (4,5,22,24,25,27,28) ARID4A AT rich interactive domain 4A (RBP1-like) (28) ARID5B AT rich interactive domain 5B (MRF1-like) (21) ASPM Asp (abnormal -

Supplementary Table 1. in Vitro Side Effect Profiling Study for LDN/OSU-0212320. Neurotransmitter Related Steroids
Supplementary Table 1. In vitro side effect profiling study for LDN/OSU-0212320. Percent Inhibition Receptor 10 µM Neurotransmitter Related Adenosine, Non-selective 7.29% Adrenergic, Alpha 1, Non-selective 24.98% Adrenergic, Alpha 2, Non-selective 27.18% Adrenergic, Beta, Non-selective -20.94% Dopamine Transporter 8.69% Dopamine, D1 (h) 8.48% Dopamine, D2s (h) 4.06% GABA A, Agonist Site -16.15% GABA A, BDZ, alpha 1 site 12.73% GABA-B 13.60% Glutamate, AMPA Site (Ionotropic) 12.06% Glutamate, Kainate Site (Ionotropic) -1.03% Glutamate, NMDA Agonist Site (Ionotropic) 0.12% Glutamate, NMDA, Glycine (Stry-insens Site) 9.84% (Ionotropic) Glycine, Strychnine-sensitive 0.99% Histamine, H1 -5.54% Histamine, H2 16.54% Histamine, H3 4.80% Melatonin, Non-selective -5.54% Muscarinic, M1 (hr) -1.88% Muscarinic, M2 (h) 0.82% Muscarinic, Non-selective, Central 29.04% Muscarinic, Non-selective, Peripheral 0.29% Nicotinic, Neuronal (-BnTx insensitive) 7.85% Norepinephrine Transporter 2.87% Opioid, Non-selective -0.09% Opioid, Orphanin, ORL1 (h) 11.55% Serotonin Transporter -3.02% Serotonin, Non-selective 26.33% Sigma, Non-Selective 10.19% Steroids Estrogen 11.16% 1 Percent Inhibition Receptor 10 µM Testosterone (cytosolic) (h) 12.50% Ion Channels Calcium Channel, Type L (Dihydropyridine Site) 43.18% Calcium Channel, Type N 4.15% Potassium Channel, ATP-Sensitive -4.05% Potassium Channel, Ca2+ Act., VI 17.80% Potassium Channel, I(Kr) (hERG) (h) -6.44% Sodium, Site 2 -0.39% Second Messengers Nitric Oxide, NOS (Neuronal-Binding) -17.09% Prostaglandins Leukotriene, -

Human Ephb3 Antibody Antigen Affinity-Purified Polyclonal Sheep Igg Catalog Number: AF5667
Human EphB3 Antibody Antigen Affinity-purified Polyclonal Sheep IgG Catalog Number: AF5667 DESCRIPTION Species Reactivity Human Specificity Detects human EphB3 in direct ELISAs and Western blots. In direct ELISAs, approximately 3% crossreactivity with recombinant mouse EphB3 is observed, and less than 1% crossreactivity with recombinant rat EphB1, recombinant human (rh) EphB2 and rhEphB4 is observed. Source Polyclonal Sheep IgG Purification Antigen Affinitypurified Immunogen Mouse myeloma cell line NS0derived recombinant human EphB3 Leu38Ala550 Accession # P54753 Formulation Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. See Certificate of Analysis for details. *Small pack size (SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. APPLICATIONS Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website. Recommended Sample Concentration Western Blot 1 µg/mL See Below DATA Western Blot Detection of Human EphB3 by Western Blot. Western blot shows lysates of SHSY5Y human neuroblastoma cell line. PVDF Membrane was probed with 1 µg/mL of Sheep AntiHuman EphB3 Antigen Affinity purified Polyclonal Antibody (Catalog # AF5667) followed by HRP conjugated AntiSheep IgG Secondary Antibody (Catalog # HAF016). A specific band was detected for EphB3 at approximately 110 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 8. PREPARATION AND STORAGE Reconstitution Sterile PBS to a final concentration of 0.2 mg/mL. Shipping The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. -

The Function of Human Epidermal Growth Factor Receptor-3 and Its Role in Tumors (Review)
ONCOLOGY REPORTS 30: 2563-2570, 2013 The function of human epidermal growth factor receptor-3 and its role in tumors (Review) QIN LI, ZHENYAN YUAN and BANGWEI CAO Department of Oncology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, P.R. China Received July 18, 2013; Accepted September 6, 2013 DOI: 10.3892/or.2013.2754 Abstract. Human epidermal growth factor receptor-3 (HER-3) 4 highly homologous members, HER-1 (ErbB1), HER-2 is the third member of the HER family. It was previously (ErbB2), HER-3 (ErbB3) and HER-4 (ErbB4). The distin- considered not to contain tyrosine kinase activity and catalytic guishing characteristics of the HER family are interdependent activity and the intracellular region of HER-3 could not bind and functional complementation between members. After ATP and be auto-phosphorylated. Thus, the clinical value the ligands bind to the receptor, it promotes the formation of of HER-3 was ignored. Currently, biochemical analysis has HER/ErbB receptor homodimer or heterodimer which leads to confirmed that the kinase domain of HER-3 is a specific allo- activation of the tyrosine kinase domain (1,2) and downstream steric activator; it acts as a functional activator to activate the signaling pathways (3,4). Signal transduction networks control recipient kinase (HER-1, HER-2, HER-4). With the in-depth cellular activities such as gene expression, mitosis, cell differ- knowledge of its structure and function, studies on the relation- entiation, cell proliferation, cell survival and apoptosis (1,5). ship of HER-3 and human tumors are rapidly increasing. HER-3 HER-3 is a distinctive member of the HER family as its is closely related to tumorigenesis, progression and metastasis. -

Human Ephb3 Antibody
Human EphB3 Antibody Monoclonal Mouse IgG2B Clone # 647308 Catalog Number: MAB5667 DESCRIPTION Species Reactivity Human Specificity Detects human EphB3 in direct ELISAs and Western blots. In Western blots, 100% cross-reactivity with recombinant mouse (rm) EphB3, 25% cross-reactivity with rmEphB2, and no cross-reactivity with recombinant human (rh) EphA1, A2, A5, A6, A10, rmEphA3, A4, A7, B4, B6, or recombinant rat EphB1. Source Monoclonal Mouse IgG2B Clone # 647308 Purification Protein A or G purified from hybridoma culture supernatant Immunogen Mouse myeloma cell line NS0-derived recombinant human EphB3 Leu38-Ala550 Accession # P54753 Formulation Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. See Certificate of Analysis for details. *Small pack size (-SP) is supplied either lyophilized or as a 0.2 μm filtered solution in PBS. APPLICATIONS Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website. Recommended Sample Concentration Western Blot 2 µg/mL See Below DATA Western Blot Detection of Human EphB3 by Western Blot. Western blot shows lysates of human cerebellum tissue. PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human EphB3 Monoclonal Antibody (Catalog # MAB5667) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # Catalog # HAF018). A specific band was detected for EphB3 at approximately 130 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1. PREPARATION AND STORAGE Reconstitution Sterile PBS to a final concentration of 0.5 mg/mL. Shipping The product is shipped at ambient temperature. -

Epha3 Function Is Regulated by Multiple
EPHA3 FUNCTION IS REGULA TED BY MULTIPLE PHOSPHOTYROSINE RESIDUES 2 1 Guanfang Shi\ Gang Yue , Mike Sheng\ Suzie Chen\ and Renping Zhou IDepartment of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ 08854; 2Department of Oral Biology, New Jersey Dental School, University of Medicine & Dentistry of New Jersey, Newark, NJ 07101. Address correspondence to: Renping Zhou, Ph. D. Phone: (732) 445-3400 ext. 264; Fax: (732) 445-0687; E-mail: rzhou @rci.rutgers.edu EphA3, a member of the Eph family of tyrosine carcinoma was significantly correlated with kinase receptors, has been implicated in a regional lymph node metastasis (5). variety of tumors including melanoma, Overexpression of ephrin-B2, a ligand for EphB glioblastoma, pancreatic carcinoma, lung and receptors, in B 16 melanoma cells enhanced colon cancers. The loss of EphA3 function has integrin-mediated ECM-attachment and migration been associated with colorectal cancer. To (6). EphB2 positively regulates glioma cell identify potential roles in tumor formation, we adhesion, growth, and invasion via R-Ras signaling examined effects of EphA3 expression and (7). In contrast to promote tumor metastasis and activation on melanoma cell migration, and cancer cell line migration, Eph receptors may also identified key signal transducer docking sites of regulate the cell motility negatively. Hafner and the receptor. This analysis showed that the coworkers compared EphB6 mRNA level in human melanoma cell line WMl15 lacks benign nevi, primary melanomas, and metastatic EphA3 expression, while two other melanoma melanomas, and found a progressive and cell lines, WM239A and C8161, both retain significant reduction of EphB6 mRNA expression expression of the receptor. -

Review Diverse Roles of Eph Receptors and Ephrins in The
Developmental Cell, Vol. 7, 465–480, October, 2004, Copyright 2004 by Cell Press Diverse Roles of Eph Receptors Review and Ephrins in the Regulation of Cell Migration and Tissue Assembly Alexei Poliakov, Marisa Cotrina, repulsion of cells, in others they promote adhesion and and David G. Wilkinson* attraction. Recent work has shown that some cells Division of Developmental Neurobiology switch between these distinct responses. This review National Institute for Medical Research will focus on developmental roles of repulsion and at- The Ridgeway, Mill Hill traction responses to Eph/ephrin activation and then London NW7 1AA discuss biochemical mechanisms that may regulate United Kingdom these diverse responses. Structure, Clustering, and Signal Transduction Eph receptor tyrosine kinases and ephrins have key Structure and Binding Specificities of Eph roles in regulation of the migration and adhesion of Receptors and Ephrins cells required to form and stabilize patterns of cell Eph receptors are transmembrane receptor tyrosine ki- organization during development. Activation of Eph nases (RTKs) with a number of distinctive features com- receptors or ephrins can lead either to cell repulsion pared with other RTKs, including the extracellular region or to cell adhesion and invasion, and recent work has comprised of an N-terminal ephrin binding domain, a found that cells can switch between these distinct re- cysteine-rich EGF-like domain, and two fibronectin type sponses. This review will discuss biochemical mecha- III motifs (Figure 1). In addition to a tyrosine kinase do- nisms and developmental roles of the diverse cell re- main, the intracellular region includes a number of con- sponses controlled by Eph receptors and ephrins. -

Low Expression of Ephb2, Ephb3, and Ephb4 in Bladder Cancer: Novel Potential Indicators of Muscular Invasion
Original Article Yonsei Med J 2021 Aug;62(8):679-690 https://doi.org/10.3349/ymj.2021.62.8.679 pISSN: 0513-5796 · eISSN: 1976-2437 Low Expression of EphB2, EphB3, and EphB4 in Bladder Cancer: Novel Potential Indicators of Muscular Invasion Tae Ho Lee1, Jin Hyung Heo2, Ju-Yeon Jeong3, Gee Hoon Lee2, Dong Soo Park1, and Tae Hoen Kim2,3 Departments of 1Urology, 2Pathology, and 3Clinical Research Institute, CHA Bundang Medical Center, CHA University, Seongnam, Korea. Purpose: Eph receptors are differentially expressed in numerous malignant tumors. This study intended to analyze the roles of EphB receptors (EphB2, B3, and B4) in urinary bladder cancer. Materials and Methods: Tissue microarray-based immunohistochemical analysis was used to investigate the expression patterns of EphB2, EphB3, and EphB4 in 154 bladder cancer specimens. Immunohistochemical staining was conducted examining the extent of stained cells and staining intensity. EphB was considered to be highly expressed when the intensity of staining was more than moderate in >25% of cells in the tissue section. Small interfering RNA (siRNA) was used to knock down EphB expression in bladder cancer cell lines (T24, 5637) to determine the effects of EphB on tumor cell invasion, proliferation, and migration. Results: EphB receptors (B2, B3, and B4) were detected in 40.9% (EphB2, 63/154), 71.4% (EphB3, 110/154), and 53.2% (EphB4, 82/154) of bladder cancer specimens. Low expression of EphB2, B3, and B4 receptors were significantly associated with higher tu- mor grade (EphB2, p<0.001; EphB3, p=0.032; EphB4, p<0.001) and muscular invasion (EphB2, p=0.002; EphB3, p=0.009; EphB4, p<0.001).