Opportunities Lost: Zoos and the Marsupial That Tried to Be a Wolf
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Hunter and Biodiversity in Tasmania
The Hunter and Biodiversity in Tasmania The Hunter takes place on Tasmania’s Central Plateau, where “One hundred and sixty-five million years ago potent forces had exploded, clashed, pushed the plateau hundreds of metres into the sky.” [a, 14] The story is about the hunt for the last Tasmanian tiger, described in the novel as: “that monster whose fabulous jaw gapes 120 degrees, the carnivorous marsupial which had so confused the early explorers — a ‘striped wolf’, ‘marsupial wolf.’” [a, 16] Fig 1. Paperbark woodlands and button grass plains near Derwent Bridge, Central Tasmania. Source: J. Stadler, 2010. Biodiversity “Biodiversity”, or biological diversity, refers to variety in all forms of life—all plants and animals, their genes, and the ecosystems they live in. [b] It is important because all living things are connected with each other. For example, humans depend on living things in the environment for clean air to breathe, food to eat, and clean water to drink. Biodiversity is one of the underlying themes in The Hunter, a Tasmanian film directed by David Nettheim in 2011 and based on Julia Leigh’s 1999 novel about the hunt for the last Tasmanian Tiger. The film and the novel showcase problems that arise from loss of species, loss of habitat, and contested ideas about land use. The story is set in the Central Plateau Conservation Area and much of the film is shot just south of that area near Derwent Bridge and in the Florentine Valley. In Tasmania, land clearing is widely considered to be the biggest threat to biodiversity [c, d]. -

Thylacinidae
FAUNA of AUSTRALIA 20. THYLACINIDAE JOAN M. DIXON 1 Thylacine–Thylacinus cynocephalus [F. Knight/ANPWS] 20. THYLACINIDAE DEFINITION AND GENERAL DESCRIPTION The single member of the family Thylacinidae, Thylacinus cynocephalus, known as the Thylacine, Tasmanian Tiger or Wolf, is a large carnivorous marsupial (Fig. 20.1). Generally sandy yellow in colour, it has 15 to 20 distinct transverse dark stripes across the back from shoulders to tail. While the large head is reminiscent of the dog and wolf, the tail is long and characteristically stiff and the legs are relatively short. Body hair is dense, short and soft, up to 15 mm in length. Body proportions are similar to those of the Tasmanian Devil, Sarcophilus harrisii, the Eastern Quoll, Dasyurus viverrinus and the Tiger Quoll, Dasyurus maculatus. The Thylacine is digitigrade. There are five digital pads on the forefoot and four on the hind foot. Figure 20.1 Thylacine, side view of the whole animal. (© ABRS)[D. Kirshner] The face is fox-like in young animals, wolf- or dog-like in adults. Hairs on the cheeks, above the eyes and base of the ears are whitish-brown. Facial vibrissae are relatively shorter, finer and fewer than in Tasmanian Devils and Quolls. The short ears are about 80 mm long, erect, rounded and covered with short fur. Sexual dimorphism occurs, adult males being larger on average. Jaws are long and powerful and the teeth number 46. In the vertebral column there are only two sacrals instead of the usual three and from 23 to 25 caudal vertebrae rather than 20 to 21. -

The Earliest Motion Picture Footage of the Last Captive Thylacine? Stephen R
The earliest motion picture footage of the last captive thylacine? Stephen R. Sleightholme1 and Cameron R. Campbell2 1 Project Director - International Thylacine Specimen Database (ITSD), 26 Bitham Mill, Westbury, BA13 3DJ, UK. E-mail: [email protected] 2 Curator of the online Thylacine Museum: http://www.naturalworlds.org/thylacine/ 8707 Eagle Mountain Circle, Fort Worth, TX 76135, USA. E-mail: [email protected] Downloaded from http://meridian.allenpress.com/australian-zoologist/article-pdf/37/3/282/1587738/az_2014_021.pdf by guest on 02 October 2021 David Fleay’s 1933 motion film footage of the last captive thylacine at the Beaumaris Zoo in Hobart was thought to be the only film record of this thylacine. The authors provide evidence to confirm that two earlier motion picture films, erroneously dated to 1928, also show the last captive specimen at the zoo. ABSTRACT Key Words: Thylacine, Thylacinus cynocephalus, motion picture, Beaumaris Zoo, David Fleay. DOI: http://dx.doi.org/10.7882/AZ.2014.021 Introduction The thylacine or Tasmanian tiger (Thylacinus cynocephalus) is the largest marsupial carnivore to have existed into modern times. The last known captive specimen, a male (Sleightholme, 2011), died at the Beaumaris Zoo on the Queen’s Domain in Hobart on the night of the 7th September 1936. There are seven historical motion picture films known to exist of captive thylacines, all of which were made between 1911 and 1933 (Fig. 1, [i-vii]) 1. Five of the films were recorded at the Beaumaris Zoo in Hobart, and the remaining two at the London Zoo, Regent’s Park. The earliest known footage was filmed by a Mr. -

The Perils of De-Extinction
MINDING NATURE 8.1 The Perils of De-extinction By BEN A. MINTEER TASMANIA’S LOST TIGER Neither tiger nor wolf, the “Thylacine” (as it became t wasn’t a tiger, at least not in the biological known after several taxonomic fits and starts), was, in sense. But in the cultural imagination of British fact, a marsupial mammal roughly the size of a hyena. It and Irish sheepherders transplanted to “Van Di- went extinct on the Australian mainland around 35,000 I emen’s Land” of the southeastern coast of Aus- years ago, a period that corresponded with the arrival of tralia in the early nineteenth century, the carnivorous, the dingo (the natural history of the species, however, is striped creature with the stealthy nature certainly ft a bit foggy). Tasmania, an island state around the size of the bill.1 Dubbed the Tasmanian tiger—or, alterna- Ireland or West Virginia, only ever held a small remnant tively, Tasmanian wolf (which it also was not)—the population of Thylacines, probably not more than five elusive animal was viewed as a threat to the island’s thousand at the time of British settlement in 1803.2 rapidly growing though ultimately ill-suited sheep in- The species would be decimated in the nineteenth dustry, an unwanted varmint that was also seen as an century by bounty hunters working at the behest of the impediment to the development of the Tasmanian wil- Van Diemen’s Land Company, a United Kingdom–based derness. wool-growing venture with a myopic desire for a pred- ator-free landscape. -

Ontogenetic Origins of Cranial Convergence Between the Extinct Marsupial Thylacine and Placental Gray Wolf ✉ ✉ Axel H
ARTICLE https://doi.org/10.1038/s42003-020-01569-x OPEN Ontogenetic origins of cranial convergence between the extinct marsupial thylacine and placental gray wolf ✉ ✉ Axel H. Newton 1,2,3 , Vera Weisbecker 4, Andrew J. Pask2,3,5 & Christy A. Hipsley 2,3,5 1234567890():,; Phenotypic convergence, describing the independent evolution of similar characteristics, offers unique insights into how natural selection influences developmental and molecular processes to generate shared adaptations. The extinct marsupial thylacine and placental gray wolf represent one of the most extraordinary cases of convergent evolution in mammals, sharing striking cranial similarities despite 160 million years of independent evolution. We digitally reconstructed their cranial ontogeny from birth to adulthood to examine how and when convergence arises through patterns of allometry, mosaicism, modularity, and inte- gration. We find the thylacine and wolf crania develop along nearly parallel growth trajec- tories, despite lineage-specific constraints and heterochrony in timing of ossification. These constraints were found to enforce distinct cranial modularity and integration patterns during development, which were unable to explain their adult convergence. Instead, we identify a developmental origin for their convergent cranial morphologies through patterns of mosaic evolution, occurring within bone groups sharing conserved embryonic tissue origins. Inter- estingly, these patterns are accompanied by homoplasy in gene regulatory networks asso- ciated with neural crest cells, critical for skull patterning. Together, our findings establish empirical links between adaptive phenotypic and genotypic convergence and provides a digital resource for further investigations into the developmental basis of mammalian evolution. 1 School of Biomedical Sciences, Monash University, Melbourne, VIC, Australia. 2 School of BioSciences, The University of Melbourne, Melbourne, VIC, Australia. -
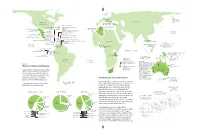
New World Extinctions: Where Next? Mammals: the Recently Departed
GERMANY BERING SEA Bavarian Commander I. pine vole 1962 EURASIA Stellerís sea cow NORTH 1768 CORSICA & SARDINIA AMERICA Sardinian pika Late 18th C. WEST INDIES CUBA MEDITERRANEAN Cuban spiny rats (2 species)* HAITI/ DOMINICAN REPUBLIC ALGERIA Hutia (6 species)* San Pedro Nolasco I. Cuban island shrews (4 species)* CANARY ISLANDS Red gazelle Arredondoís solenodon* Quemi* Volcano mouse* Late 19th c. Pembertonís Hispaniolan spiny rats (2 species)* deer mouse 1931 CAYMAN IS. Hispaniolan island shrews (3 species)* PACIFIC Cayman island shrews Marcanoís solenodon* OCEAN (2 species)* Maria Madre I. PUERTO RICO Nelsonís rice rat 1897 Cayman coneys (two species)* Puerto Rican spiny rats (2 species)* Cayman hutia* MEXICO Omilteme cottontail 1991 BARBUDA Barbuda muskrat* CARIBBEAN Little Swan I. Caribbean MARTINIQUE Martinique muskrat 1902 Little Swan Island coney 1950s-60s PHILIPPINES: Ilin I. Monk seal BARBADOS Barbados rice rat 1847-1890 AFRICA J AMAICA 1950s Small Ilin cloud rat 1953 Jamaican rice rat 1877 ST. VINCENT GUAM Jamaican monkey* St. Vincent rice rat 1890s Guam flying fox 1967 PACIFIC ST. LUCIA PHILIPPINES: Negros I. GHANA Philippine bare- OCEAN St. Lucia muskrat Groove-toothed forest CAROLINES: Palau I. pre-1881 backed fruit bat Palau flying fox 1874 mouse 1890 1975 GAL¡PAGOS ISLANDS San Salvador I. INDIAN OCEAN San Salvador rice rat 1965 Santa Cruz I. Curioís large rice rat* SOLOMON ISLANDS: Guadalcanal Isabela I. Gal·pagos rice rat Little pig rat 1887 Large rice rat* 1930s TANZANIA Giant naked-tailed rat 1960s Small rice rats (2 species)* Painted bat 1878 NEW GUINEA CHRISTMAS I. Large-eared Maclearís rat 1908 nyctophilus (bat) 1890 MADAGASCAR Bulldog rat 1908 Santa Cruz I. -

Disclosure and Revision in Photographs of the Thylacine
Society and Animals 15 (2007) 241-256 www.brill.nl/soan Imaging Extinction: Disclosure and Revision in Photographs of the Th ylacine (Tasmanian tiger) Carol Freeman Honorary Research Associate, School of Geography & Environmental Studies, University of Tasmania, Private Bag 78, HOBART Tasmania 7001 Email [email protected] Received 15 December 2006, Accepted 14 March 2007 Abstract Th e thylacine was a shy and elusive nonhuman animal who survived in small numbers on the island of Tasmania, Australia, when European settlers arrived in 1803. After a deliberate cam- paign of eradication, the species disappeared 130 years later. Visual and verbal constructions in the nineteenth century labeled the thylacine a ferocious predator, but photographs of individuals in British and American zoos that were used to illustrate early twentieth-century zoological works presented a very different impression of the animal. Th e publication of these photographs, however, had little effect on the relentless progress of extermination. Th is essay focuses on the relationship between photographs of thylacines and the process of extinction, between images and words, and between pictures of dead animals and live ones. Th e procedures, claims, and limitations of photography are crucial to the messages generated by these images and to the role they played in the representation of the species. Th is essay explains why the medium of photog- raphy and pleas for preservation could not save the thylacine. Keywords thylacine, representation, photography, human-animal interactions, extinction Introduction References to photography in nineteenth-century zoological publications often stress its difference from previous picture-making traditions; for instance, naturalist Agassiz (1871) commented that “the accuracy of a photographic illustration is of course far beyond that of an engraving or lithograph” (p. -

Thylacine Conspiracy
Thylacine Conspiracy Bill Cromer \ 2 The thylacine (pronounced 'thigh-la-seen'), also known as the Tasmanian Tiger or Tasmanian Wolf, is – or was – the world's largest marsupial carnivore ( marsupial : a mammal with a pouch for carrying young; carnivore : a meat-eater). Bill Cromer Thylacine Conspiracy 3 Acknowledgments I am delighted and grateful that Eric Guiler read the manuscript, and has checked the scientific details of the thylacine - as far as they are known. Dr. Guiler is the recognized world expert on the thylacine, the author of Thylacine: The tragedy of the Tasmanian Tiger and related scientific articles, the organizer of and participant in several searches for the elusive animal, and until recently a firm believer in its continued existence. Chris MacGeorge of the Bureau of Meteorology in Hobart went out of his way to help me with weather patterns and rainfall figures for northeastern Tasmania. For an insight into how trustees operate, and especially how they handle hard-to-find beneficiaries, I am indebted to Ken Lord. Veterinarian Barry Springfield kindly answered my curious-sounding questions about how someone could keep a 'dog-like animal' sedated for several days. Rob Usher talked on my behalf to two private Cessna Citation pilots and discussed with them - hypothetically, of course - a realistic flight plan for smuggling a Tasmanian Tiger across the Pacific Ocean. Janet Terry, an Internet-met fellow writer in the United States, kindly critiqued the manuscript and helped iron out the wrinkles in my narrative. My wife Hilary was, as always, encouraging, and uncomplaining about my early-morning forays with the word processor. -

After the Thylacine: in Pursuit of Cinematic and Literary Improvised Encounters with the Extinct
Liminalities: A Journal of Performance Studies Vol. 14, No. 1 (2018) After the Thylacine: In Pursuit of Cinematic and Literary Improvised Encounters with the Extinct Nicholas Chare Introduction: Tainted Evidence A coughing bark was one of its forms of vocal expression.1 Or, more precisely, coughing bark is how one of its forms of vocal expression has been styled, part- imitated, translated into words; circumscribed for the purposes of description, classification, possession and preservation. The coughing bark, carrying under- tones of canine sickness, refers to a non-human vocalisation, a vocalisation made by Thylacinus cynocephalus, otherwise known as the thylacine or the Tasmanian tiger. This non-human animal, a carnivorous marsupial, was officially declared extinct by the International Union for the Conservation of Nature in 1982. It was also acknowledged as extinct by the Tasmanian Government in 1986. Alt- hough the thylacine was once native to mainland Australia and Papua New Guinea it likely died out in those regions around 2000 years ago and the animal is therefore most associated with the island of Tasmania where a relictual popu- lation survived into the twentieth-century.2 The last officially recorded thylacine in Tasmania, commonly referred to as “Benjamin”, died at Hobart Zoo in 1936.3 Nicholas Chare is Associate Professor of Modern Art in the Department of History of Art and Film Studies at the Université de Montréal. He is the co-author (with Dominic Williams) of Matters of Testimony: Interpreting the Scrolls of Auschwitz (2016) and the author of After Francis Bacon: Synaesthesia and Sex in Paint (2012). He has also co-edited (with Ika Willis) a special issue of the journal parallax on the theme of Trans-: Across/Beyond (2016). -

Marsupial Carnivore Feeding Ecology and Extinction Risk
WHO'S ON THE MENU: MARSUPIAL CARNIVORE FEEDING ECOLOGY AND EXTINCTION RISK Thesis submitted by ARIE TTARD M A For the Degree of Doctor of Philosophy in the School of Biological, Earth & Environmental Sciences Faculty of Science March 2013 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: Attard First name: Marie Other name/s: Rosanna Gabrielle Abbreviation for degree as given in the University calendar: PhD School: School of Biological, Earth and Environmental Sciences Faculty: Faculty of Science Title: Who's on the menu: marsupial carnivore feeding ecology and extinction risk Abstract The aim of this thesis is to assess the role of diet in the extinction of Australia's iconic marsupial carnivore, the thylacine (Thylacinus cynocephalus) in Tasmania. Herein, we present two novel techniques to address fundamental questions regarding their maximum prey size and potential competition with sympatric predators. Three-dimensional computer models of the thylacine skull were used to assess their biomechanical limitations in prey size within a comparative context. This included living relatives from the family Dasyuiridae as well as a recently recovered fossil, Nimbacinus dickoni, from the family Thylacindae. Stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) of tissues from thylacine and potential prey species were used to assess the thylacine’s dietary composition. Furthermore, we integrate historical and recent marsupial carnivore stable isotope data to assess long-term changes in the ecosystem in response to multiple human impacts following European settlement. Our biomechanical findings support the notion that solitary thylacines were limited to hunting prey weighing less than their body mass. -

Proceedings of the Royal Zoological Society of New South Wales
QL . fcqA-4- 1 R885Z PROCEEDINGS OP THE Royal Zoological Society OP New South Wales FOR THE YEAR 1938-9. AUGUST 24, 1939. SYDNEY: Published by the Society, 28 Martin Place. LONDON: Wheldon & Wesley Limited, 2, 3 and 4 Arthur Street, New Oxford Street, W.C.2. ROYAL ZOOLOGICAL SOCIETY OF NEW SOUTH WALES (Established 1879.) Registered under the Companies Act, 1899 (1917). PATRONS His Excellency the Governor of New South Wales, The Lord Wakehurst, K.C.M.G. Sir Philip Woolcott Game, G.B.E., K.C.B., D.S.O. COUNCIL, 1939-40. President: Keith A. Hindwood, C.F.A.O.U. Vice-Presidents: A. F. Basset Hull, M.B.E., F.R.Z.S.; Albert Littlejohn; Noel L. Roberts; and Gilbert P. Whitley, F.R.Z.S. Honorary Secretary: Tom Iredale. Honorary Treasurer: Phillip Shipway Honorary Librarian: A. S. Le Souef, CJM.Z.S. Members: E. J. Bryce, F.R.G.S.; Neville W. Cayley, F.R.Z.S.; Aubrey Halloran, B.A., LL.B.; Frank Marshall, C.M.G., D.D.S.; Garnet Halloran, B.Sc, M.D., F.R.A.C.S., F.R.C.S. (Ed.); Charles F. Laseron; Michael S. R. Sharland; Theodore Cleveland Roughley, B.Sc; J. R. Wallace; and Emil H. Zeck. Assistant Honorary Secretary: Betty French. Honorary Auditor: R. J. Stiffe, A.C.A. (Aust.). OFFICERS OF SECTIONS. Avicultural Section. Chairman: A. H. Brain. Hon. Secretary: John D. Whaling. Budgerigar Section. Chairman: R. J. Murray. Hon. Secretary: F. Brennan. Marine Zoological Section. Chairman: G. P. Whitley. Hon. Secretary: W. E. -

(Dasyuromorphia: Thylacinidae) and the Evolutionary Context of the Modern Thylacine
The pre-Pleistocene fossil thylacinids (Dasyuromorphia: Thylacinidae) and the evolutionary context of the modern thylacine Douglass S. Rovinsky1, Alistair R. Evans2,3 and Justin W. Adams1 1 Department of Anatomy and Developmental Biology, Monash University, Clayton, VIC, Australia 2 School of Biological Sciences, Monash University, Clayton, VIC, Australia 3 Geosciences, Museums Victoria, Melbourne, VIC, Australia ABSTRACT The thylacine is popularly used as a classic example of convergent evolution between placental and marsupial mammals. Despite having a fossil history spanning over 20 million years and known since the 1960s, the thylacine is often presented in both scientific literature and popular culture as an evolutionary singleton unique in its morphological and ecological adaptations within the Australian ecosystem. Here, we synthesise and critically evaluate the current state of published knowledge regarding the known fossil record of Thylacinidae prior to the appearance of the modern species. We also present phylogenetic analyses and body mass estimates of the thylacinids to reveal trends in the evolution of hypercarnivory and ecological shifts within the family. We find support that Mutpuracinus archibaldi occupies an uncertain position outside of Thylacinidae, and consider Nimbacinus richi to likely be synonymous with N. dicksoni. The Thylacinidae were small-bodied (< ~8 kg) unspecialised faunivores until after the ~15–14 Ma middle Miocene climatic transition (MMCT). After the MMCT they dramatically increase in size and develop adaptations to a hypercarnivorous diet, potentially in response to the aridification of Submitted 27 March 2019 the Australian environment and the concomitant radiation of dasyurids. This fossil Accepted 10 July 2019 history of the thylacinids provides a foundation for understanding the ecology of the Published 2 September 2019 modern thylacine.