A Salmon Ecological Systems Model Nushagak Kvichak, Bristol Bay 2014
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

SUSTAINABLE FISHERIES and RESPONSIBLE AQUACULTURE: a Guide for USAID Staff and Partners
SUSTAINABLE FISHERIES AND RESPONSIBLE AQUACULTURE: A Guide for USAID Staff and Partners June 2013 ABOUT THIS GUIDE GOAL This guide provides basic information on how to design programs to reform capture fisheries (also referred to as “wild” fisheries) and aquaculture sectors to ensure sound and effective development, environmental sustainability, economic profitability, and social responsibility. To achieve these objectives, this document focuses on ways to reduce the threats to biodiversity and ecosystem productivity through improved governance and more integrated planning and management practices. In the face of food insecurity, global climate change, and increasing population pressures, it is imperative that development programs help to maintain ecosystem resilience and the multiple goods and services that ecosystems provide. Conserving biodiversity and ecosystem functions are central to maintaining ecosystem integrity, health, and productivity. The intent of the guide is not to suggest that fisheries and aquaculture are interchangeable: these sectors are unique although linked. The world cannot afford to neglect global fisheries and expect aquaculture to fill that void. Global food security will not be achievable without reversing the decline of fisheries, restoring fisheries productivity, and moving towards more environmentally friendly and responsible aquaculture. There is a need for reform in both fisheries and aquaculture to reduce their environmental and social impacts. USAID’s experience has shown that well-designed programs can reform capture fisheries management, reducing threats to biodiversity while leading to increased productivity, incomes, and livelihoods. Agency programs have focused on an ecosystem-based approach to management in conjunction with improved governance, secure tenure and access to resources, and the application of modern management practices. -

Alaska Department of Fish and Game Staff Comments on Subsistence, Personal Use, Sport, Guided Sport, and Commercial Finfish Regu
ALASKA DEPARTMENT OF FISH AND GAME STAFF COMMENTS ON SUBSISTENCE, PERSONAL USE, SPORT, GUIDED SPORT, AND COMMERCIAL FINFISH REGULATORY PROPOSALS FOR THE ARCTIC-YUKON-KUSKOKWIM AREA FINFISH ALASKA BOARD OF FISHERIES MEETING FAIRBANKS, ALASKA JANUARY 12-19 , 2004 The following staff comments were prepared by the Alaska Department of Fish and Game for use at the Alaska Board of Fisheries (Board) meeting, January 12-19, 2004 in Fairbanks, Alaska. The comments are forwarded to assist the public and Board. The comments contained herein should be considered preliminary and subject to change, as new information becomes available. Final department positions will be formulated after review of written and oral public testimony presented to the Board. This book is organized by Board Subcommittee. A listing of staff comment page numbers by proposal number appears after the Table of Contents on page vi. The Alaska Department of Fish and Game administers all programs and activities free from discrimination based on race, color, national origin, age, sex, religion, marital status, pregnancy, parenthood, or disability. The department administers all programs and activities in compliance with Title VI of the Civil Rights Act of 1964, Section 504 of the Rehabilitation Act of 1973, Title II of the Americans with Disabilities Act of 1990, the Age Discrimination Act of 1975, and Title IX of the Education Amendments of 1972. If you believe you have been discriminated against in any program, activity, or facility, or if you desire further information, please write to ADF&G, P.O. Box 25526, Juneau, AK 99802-5526; U.S. Fish and Wildlife Service, 4040 N. -

Bristol Bay, Alaska
EPA 910-R-14-001C | January 2014 An Assessment of Potential Mining Impacts on Salmon Ecosystems of Bristol Bay, Alaska Volume 3 – Appendices E-J Region 10, Seattle, WA www.epa.gov/bristolbay EPA 910-R-14-001C January 2014 AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J U.S. Environmental Protection Agency Region 10 Seattle, WA CONTENTS VOLUME 1 An Assessment of Potential Mining Impacts on Salmon Ecosystems of Bristol Bay, Alaska VOLUME 2 APPENDIX A: Fishery Resources of the Bristol Bay Region APPENDIX B: Non-Salmon Freshwater Fishes of the Nushagak and Kvichak River Drainages APPENDIX C: Wildlife Resources of the Nushagak and Kvichak River Watersheds, Alaska APPENDIX D: Traditional Ecological Knowledge and Characterization of the Indigenous Cultures of the Nushagak and Kvichak Watersheds, Alaska VOLUME 3 APPENDIX E: Bristol Bay Wild Salmon Ecosystem: Baseline Levels of Economic Activity and Values APPENDIX F: Biological Characterization: Bristol Bay Marine Estuarine Processes, Fish, and Marine Mammal Assemblages APPENDIX G: Foreseeable Environmental Impact of Potential Road and Pipeline Development on Water Quality and Freshwater Fishery Resources of Bristol Bay, Alaska APPENDIX H: Geologic and Environmental Characteristics of Porphyry Copper Deposits with Emphasis on Potential Future Development in the Bristol Bay Watershed, Alaska APPENDIX I: Conventional Water Quality Mitigation Practices for Mine Design, Construction, Operation, and Closure APPENDIX J: Compensatory Mitigation and Large-Scale Hardrock Mining in the Bristol Bay Watershed AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J Appendix E: Bristol Bay Wild Salmon Ecosystem: Baseline Levels of Economic Activity and Values Bristol Bay Wild Salmon Ecosystem Baseline Levels of Economic Activity and Values John Duffield Chris Neher David Patterson Bioeconomics, Inc. -

Snohomish River Basin Salmon Conservation Plan: Status and Trends
SNOHOMISH RIVER BASIN SALMON CONSERVATION PLAN STATUS AND TRENDS DECEMBER 2019 CONTENTS Introduction 1 Status Update 3 Snohomish Basin 3 Salmon Plan Overview 7 Implementation Progress 10 Status and Trends 18 Salmon in the Basin 18 Chinook Salmon 19 Coho Salmon 22 Steelhead 24 Bull Trout 26 Chum Salmon 27 Factors Affecting Survival of Salmon Species 29 Basin Trends 32 Salmon Plan Implementation 46 Habitat Restoration Progress 48 Restoration Funding 67 Habitat and Hydrology Protection Observations 70 Monitoring and Adaptive Management 76 Updating the Basin-Wide Vision for Recovery 77 Considerations for a Changing Future 78 Restoration Opportunities and Challenges 82 Integration of Habitat, Harvest, and Hatchery Actions Within the Basin 86 Multi-Objective Planning 88 Updating Our Salmon Plan 90 Credits and Acknowledgments 93 INTRODUCTION Since 1994 — 5 years before the Endangered Species Act (ESA) listing of Chinook salmon — partner organizations in the Snohomish Basin have been coordinating salmon recovery efforts to improve salmon stock numbers. In 2005, the Snohomish Basin Salmon Recovery Forum (Forum) adopted the Snohomish River Basin Salmon Conservation Plan1 (Salmon Plan), defining a multi-salmon strategy for the Snohomish Basin that emphasizes two ESA-listed species, Chinook salmon and bull trout, and the non-listed coho salmon. These species are used as proxies for other Basin salmon to help prevent future listings. The Salmon Plan, developed by the 41-member Forum, incorporates habitat, harvest, and hatchery management actions to bring the listed wild stocks back to healthy, harvestable levels. 1 Snohomish Basin Salmon Recovery Forum, 2005. Snohomish River Basin Salmon Conservation Plan. Snohomish County Department of Public Works, Surface Water Management Division. -

Assessment of Coastal Water Resources and Watershed Conditions at Katmai National Park and Preserve (Alaska)
National Park Service U.S. Department of the Interior Natural Resources Program Center Assessment of Coastal Water Resources and Watershed Conditions at Katmai National Park and Preserve (Alaska) Natural Resource Technical Report NPS/NRWRD/NRTR—2007/372 Cover photo: Glacier emerging from the slopes of Mt Douglas toward the Katmai coastline. August 2005. Photo: S.Nagorski 2 Assessment of Coastal Water Resources and Watershed Conditions at Katmai National Park and Preserve (Alaska) Natural Resource Technical Report NPS/NRWRD/NRTR-2007/372 Sonia Nagorski Environmental Science Program University of Alaska Southeast Juneau, AK 99801 Ginny Eckert Biology Program University of Alaska Southeast Juneau, AK 99801 Eran Hood Environmental Science Program University of Alaska Southeast Juneau, AK 99801 Sanjay Pyare Environmental Science Program University of Alaska Southeast Juneau, AK 99801 This report was prepared under Task Order J9W88050014 of the Pacific Northwest Cooperative Ecosystem Studies Unit (agreement CA90880008) Water Resources Division Natural Resource Program Center 1201 Oakridge Drive, Suite 250 Fort Collins, CO 80525 June 2007 U.S. Department of Interior Washington, D.C. 3 The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the audience, and is designed and published in a professional manner. The Natural Resource Technical Reports series is used to disseminate the peer-reviewed results of scientific studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. -

Abstract Book
January 21-25, 2013 Alaska Marine Science Symposium hotel captain cook & Dena’ina center • anchorage, alaska Bill Rome Glenn Aronmits Hansen Kira Ross McElwee ShowcaSing ocean reSearch in the arctic ocean, Bering Sea, and gulf of alaSka alaskamarinescience.org Glenn Aronmits Index This Index follows the chronological order of the 2013 AMSS Keynote and Plenary speakers Poster presentations follow and are in first author alphabetical order according to subtopic, within their LME category Editor: Janet Duffy-Anderson Organization: Crystal Benson-Carlough Abstract Review Committee: Carrie Eischens (Chair), George Hart, Scott Pegau, Danielle Dickson, Janet Duffy-Anderson, Thomas Van Pelt, Francis Wiese, Warren Horowitz, Marilyn Sigman, Darcy Dugan, Cynthia Suchman, Molly McCammon, Rosa Meehan, Robin Dublin, Heather McCarty Cover Design: Eric Cline Produced by: NOAA Alaska Fisheries Science Center / North Pacific Research Board Printed by: NOAA Alaska Fisheries Science Center, Seattle, Washington www.alaskamarinescience.org i ii Welcome and Keynotes Monday January 21 Keynotes Cynthia Opening Remarks & Welcome 1:30 – 2:30 Suchman 2:30 – 3:00 Jeremy Mathis Preparing for the Challenges of Ocean Acidification In Alaska 30 Testing the Invasion Process: Survival, Dispersal, Genetic Jessica Miller Characterization, and Attenuation of Marine Biota on the 2011 31 3:00 – 3:30 Japanese Tsunami Marine Debris Field 3:30 – 4:00 Edward Farley Chinook Salmon and the Marine Environment 32 4:00 – 4:30 Judith Connor Technologies for Ocean Studies 33 EVENING POSTER -
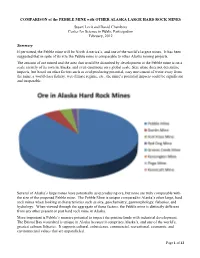
COMPARISON of the PEBBLE MINE with OTHER ALASKA LARGE HARD ROCK MINES
COMPARISON of the PEBBLE MINE with OTHER ALASKA LARGE HARD ROCK MINES Stuart Levit and David Chambers Center for Science in Public Participation February, 2012 Summary If permitted, the Pebble mine will be North America’s, and one of the world’s largest mines. It has been suggested that in spite of its size the Pebble mine is comparable to other Alaska mining projects. The amount of ore mined and the area that would be disturbed by development at the Pebble mine is on a scale entirely of its own in Alaska, and even enormous on a global scale. Size alone does not determine impacts, but based on other factors such as acid producing potential, easy movement of water away from the mine, a world class fishery, wet climate regime, etc., the mine’s potential impacts could be significant and irreparable. Several of Alaska’s large mines have potentially acid producing ore, but none are truly comparable with the size of the proposed Pebble mine. The Pebble Mine is unique compared to Alaska’s other large, hard rock mines when looking at characteristics such as size, geochemistry, geomorphology, fisheries, and hydrology. When viewed through the aggregate of these factors, the Pebble mine is distinctly different from any other present or past hard rock mine in Alaska. More important is Pebble’s massive potential to impact the pristine lands with industrial development. The Bristol Bay watershed is unique in Alaska because it comprises Alaska’s, and one of the world’s, greatest salmon fisheries. It supports cultural, subsistence, commercial, recreational, economic, and environmental values that are unparalleled. -

Regulatory Impacts on a Yup'ik Fish Camp in Southwest Alaska
Regulatory Impacts on a Yup’ik Fish Camp in Southwest Alaska by Jory Stariwat B.A., University of Alaska, Anchorage, 2008 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Anthropology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) AUGUST 2016 © Jory Stariwat, 2016 Abstract Yup’ik fishers on the Nushagak River of Southwest Alaska harvest salmon for both subsistence and commercial purposes, however their cultural protocol and formal resource management principles are unrecognized by the State of Alaska. Drawing from two summers of ethnographic research and experience as an Alaska Department of Fish & Game (ADF&G) anthropologist, I examine one state regulation preventing drift gillnetting for subsistence purposes. The analysis reveals that the Alaska Department of Fish & Game is currently preventing cultural adaptation on the Nushagak River despite Yup’ik communities maintaining sustainable harvest levels for millennia. Changes in river conditions, namely the location of sandbars and channels, in addition to warming water temperatures, necessitate the application of the traditional harvest method, drift gillnetting, to meet the harvest goals of Yup’ik fishers at the Lewis Point fish camp on the Nushagak River. The Alaska Board of Fisheries has maintained that drifting only be employed in the commercial fishery, not the subsistence fishery, despite policy dictating a subsistence priority over other consumptive uses. While failing to meet the subsistence priority codified in its own policy, the State of Alaska also fails to provide a meaningful role to the tribes in the decision-making domain of resource management. -

Petition to List the Iliamna Lake Seal, a Distinct Population Segment of Eastern North Pacific Harbor Seal (Phoca Vitulina Richardii), Under the U.S
Before the Secretary of Commerce Petition to List the Iliamna Lake Seal, a Distinct Population Segment of Eastern North Pacific Harbor Seal (Phoca vitulina richardii), under the U.S. Endangered Species Act Photo Credit: NOAA Fisheries/Dave Withrow Center for Biological Diversity 6 February 2020 i Notice of Petition Wilbur Ross, Secretary of Commerce U.S. Department of Commerce 1401 Constitution Ave. NW Washington, D.C. 20230 Email: [email protected], [email protected] Dr. Neil Jacobs, Acting Under Secretary of Commerce for Oceans and Atmosphere U.S. Department of Commerce 1401 Constitution Ave. NW Washington, D.C. 20230 Email: [email protected] Petitioner: Kristin Carden, Oceans Program Scientist, on behalf of the Center for Biological Diversity 1212 Broadway #800 Oakland, CA 94612 Phone: 510.844.7100 x327 Email: [email protected] On November 19, 2012, the Center for Biological Diversity (Center, Petitioner) submitted to the Secretary of Commerce and the National Oceanographic and Atmospheric Administration (NOAA) through the National Marine Fisheries Service (NMFS) a petition to list the Iliamna Lake population of eastern North Pacific harbor seal (Phoca vitulina richardii) as threatened or endangered under the U.S. Endangered Species Act (ESA). (See generally Center 2012.) On May 17, 2013, NMFS issued a positive 90- day finding “that the petition present[ed] substantial scientific or commercial information indicating that the petition action may be warranted” and initiated a status review. (78 Fed. Reg. 29,098 (May 17, 2013).). On November 17, 2016, NMFS issued a determination that listing was not warranted because “the seals in Iliamna Lake do not constitute a species, subspecies, or distinct population segment (DPS) under the ESA.” (81 Fed. -

Walrus Hunting at Togiak, Bristol Bay, Soutwest Alaska
WALRUS HUNTING AT TOGIAK, BRISTOL BAY, SOUTHWEST ALASKA James A. Fall, Molly Chythlook, Janet Schichnes, and Rick Sinnott’ Technical Paper No. 212 ’ Fall: Division of Subsistence, Anchorage Chythlook and Schichnes: Division of Subsistence, Dillingham Sinnott: Division of Wildlife Conservation, Anchorage Alaska Department of Fish and Game Division of Subsistence Juneau, Alaska October 1991 The Alaska Department of Fish and Game operates all of its public programs and activities free from discrimination on the basis of race, religion, color, national origin, age, sex, or handicap. Because the department receives federal funding, any person who believes he or she has been discriminated against should write to: O.E.D. U.S. Department of the Interior Washington, D.C. 20240 , ABSTRACT The report provides an overview of historic and contemporary uses of Pacific walrus in the Bristol Bay region of southwest Alaska. It focuses on the community of Togiak (population 613) and its traditional use areas, including Round Island (Yup’ik Qayaciq, “place to go in a kayak”). As part of the Walrus Islands State Game Sanctuary, Round Island, a critical haul out site, has been closed to walrus hunting since 1960. In 1991, the Togiak Traditional Council submitted a proposal to the Alaska Board of Game to allow a limited hunt for 10 walrus on Round Island in October. Hunting of walrus and other marine mammals in western Bristol Bay, including Round Island, by the Native people of the Togiak area over the last 2,500 years is documented by archaeological and ethnohistorical evidence. Until the late 1930s and early 1940% well-organized groups of hunters from Togiak traveled in kayaks to Round Island (the most reliable hunting location) and other islands armed with spears and harpoons to harvest walrus. -

ORPC Rivgen Wake Characterization
ORPC RivGen Wake Characterization Maricarmen Guerra Paris* Jim Thomson University of Washington University of Washington Seattle, WA, USA Seattle, WA, USA *Corresponding author: [email protected] in turbulence due to eddies shed by turbine blades; and iii) complex interactions between natural and turbine ABSTRACT induced turbulent structures [6]. In this investigation we assess the wake formed behind Baseline and post-deployment flow conditions were mea- a horizontal cross-flow turbine installed on the Kvichak sured at the ORPC RivGen turbine site on the Kvichak river in southwest Alaska, USA, just downstream of the river in the vicinity of Igiugig village, Alaska. Mean sur- village of Igiugig. The small village is home to 70 peo- face flow and turbulence measurements were collected ple and its electricity source currently depends on an from a drifting platform equipped with a Nortek Signa- isolated power grid fed by diesel generators. The Ocean ture 1000Hz five beam AD2CP. Baseline measurements Renewable Power Company (ORPC) has set a pilot hy- indicate a maximum flow of 2.5 m/s and a 10% turbu- drokinetic energy project on the Kvichak river stream to lent intensity in the turbine vicinity. Measurements af- provide Igiugig with a renewable and locally produced ter turbine deployment and grid connection show a sig- source of energy. nificant decrease in surface velocity up to 200 m down- ORPCs RivGen turbine was successfully deployed, stream from the turbine and an increase in turbulence tested and connected to the local power grid during intensity up to 20% that extends about 75 m down- the summers of 2014 and 2015. -

Fish Surveys in Headwater Streams of the Nushagak and Kvichak River Drainages Bristol Bay, Alaska, 2008 - 2010
FISH SURVEYS IN HEADWATER STREAMS OF THE NUSHAGAK AND KVICHAK RIVER DRAINAGES BRISTOL BAY, ALASKA, 2008 - 2010 December, 2010 ©Bridget Besaw/TNC Prepared for by Dr. Carol Ann Woody & Sarah Louise O’Neal Fisheries Research and Consulting Anchorage, Alaska PREFACE The Nature Conservancy is an international not-for profit organization with a mission to preserve the biodiversity of the earth. Several years ago the Conservancy refocused programs to advancing local conservation efforts that contribute most to protecting globally significant strongholds of biodiversity. The Alaska Chapter determined that the loss of wild Pacific salmon productivity in Alaska would have a global impact because wild salmon have been severely compromised in other parts of the world. A focus on wild salmon in Alaska inevitably leads to Bristol Bay – home to the world’s largest remaining salmon runs. In the late 1990s the Conservancy began developing partnerships with local organizations to protect the long term viability of Bristol Bay’s salmon resource. A partnership with the Curyung Tribe of Dillingham, the Bristol Bay Native Association and the Nushagak- Mulchatna Watershed Council led to the development and the publication in 2007 of The Nushagak River Watershed Traditional Use Area Conservation Plan. During the time the Conservancy was working with this partnership, the discovery of a large copper and gold ore body on state lands in the watersheds of the Nushagak and Kvichak Rivers was announced. A flurry of new mining claims followed. The discovery, now known as the Pebble Prospect, is under active exploration and environmental assessment by a consortium of mining interests. The Anadromous Fish Act (AS 16.05.871) is the key State of Alaska statutory protection for freshwater habitats of fish in Alaska.