BACTERIAL RESISTANCE in STOMATITIS and PNEUMONIA of Boa Constrictor
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Snakes: Cultural Beliefs and Practices Related to Snakebites in a Brazilian Rural Settlement Dídac S Fita1, Eraldo M Costa Neto2*, Alexandre Schiavetti3
Fita et al. Journal of Ethnobiology and Ethnomedicine 2010, 6:13 http://www.ethnobiomed.com/content/6/1/13 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access ’Offensive’ snakes: cultural beliefs and practices related to snakebites in a Brazilian rural settlement Dídac S Fita1, Eraldo M Costa Neto2*, Alexandre Schiavetti3 Abstract This paper records the meaning of the term ‘offense’ and the folk knowledge related to local beliefs and practices of folk medicine that prevent and treat snake bites, as well as the implications for the conservation of snakes in the county of Pedra Branca, Bahia State, Brazil. The data was recorded from September to November 2006 by means of open-ended interviews performed with 74 individuals of both genders, whose ages ranged from 4 to 89 years old. The results show that the local terms biting, stinging and pricking are synonymous and used as equivalent to offending. All these terms mean to attack. A total of 23 types of ‘snakes’ were recorded, based on their local names. Four of them are Viperidae, which were considered the most dangerous to humans, besides causing more aversion and fear in the population. In general, local people have strong negative behavior towards snakes, killing them whenever possible. Until the antivenom was present and available, the locals used only charms, prayers and homemade remedies to treat or protect themselves and others from snake bites. Nowadays, people do not pay attention to these things because, basically, the antivenom is now easily obtained at regional hospitals. It is under- stood that the ethnozoological knowledge, customs and popular practices of the Pedra Branca inhabitants result in a valuable cultural resource which should be considered in every discussion regarding public health, sanitation and practices of traditional medicine, as well as in faunistic studies and conservation strategies for local biological diversity. -

Spilotes Pullatus (Tiger Rat Snake Or Clibo)
UWI The Online Guide to the Animals of Trinidad and Tobago Diversity Spilotes pullatus (Tiger Rat Snake or Clibo) Family: Colubridae (Typical Snakes) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Tiger rat snake, Spilotes pullatus. [http://www.theonlinezoo.com/pages/tropical_rat_snake.html, downloaded 18 October 2016] TRAITS. Amongst the largest snakes of the Americas, with a maximum length of 4.2m (Primareptilia, 2016). The usual maximum length is 3m in males and 2.5m in females. They are long and slender with a head that is distinct from the (Trinidad-Tobagoherps, 2016). The coloration of their scales is dependent upon where they are found. However, throughout their wide range the main colour for this species is black with yellowish markings as bands (Fig. 1), diagonals or even netlike patterns (Captivebredreptileforums, 2012). Spilotes pullatus is a non-venomous snake. DISTRIBUTION. Spilotes pullatus can be found from southern Mexico and other countries south to Paraguay, including Trinidad and Tobago (Fig. 2). HABITAT AND ECOLOGY. Can be found in abundance in habitats close to water, mainly forested areas (Littlescorpion, 2016). They are diurnal semi-arboreal snakes, using both trees and UWI The Online Guide to the Animals of Trinidad and Tobago Diversity the ground, and can be found basking during the day on branches (Trinidad-Tobagoherps, 2016). They feed on a variety of rodents, bats, eggs and small birds, occasionally on amphibians and reptiles. Unlike other species of non-venomous snakes, their prey are not killed by being coiled around but by biting or holding and pressing against a solid surface or object. -

Notice Warning Concerning Copyright Restrictions P.O
Publisher of Journal of Herpetology, Herpetological Review, Herpetological Circulars, Catalogue of American Amphibians and Reptiles, and three series of books, Facsimile Reprints in Herpetology, Contributions to Herpetology, and Herpetological Conservation Officers and Editors for 2015-2016 President AARON BAUER Department of Biology Villanova University Villanova, PA 19085, USA President-Elect RICK SHINE School of Biological Sciences University of Sydney Sydney, AUSTRALIA Secretary MARION PREEST Keck Science Department The Claremont Colleges Claremont, CA 91711, USA Treasurer ANN PATERSON Department of Natural Science Williams Baptist College Walnut Ridge, AR 72476, USA Publications Secretary BRECK BARTHOLOMEW Notice warning concerning copyright restrictions P.O. Box 58517 Salt Lake City, UT 84158, USA Immediate Past-President ROBERT ALDRIDGE Saint Louis University St Louis, MO 63013, USA Directors (Class and Category) ROBIN ANDREWS (2018 R) Virginia Polytechnic and State University, USA FRANK BURBRINK (2016 R) College of Staten Island, USA ALISON CREE (2016 Non-US) University of Otago, NEW ZEALAND TONY GAMBLE (2018 Mem. at-Large) University of Minnesota, USA LISA HAZARD (2016 R) Montclair State University, USA KIM LOVICH (2018 Cons) San Diego Zoo Global, USA EMILY TAYLOR (2018 R) California Polytechnic State University, USA GREGORY WATKINS-COLWELL (2016 R) Yale Peabody Mus. of Nat. Hist., USA Trustee GEORGE PISANI University of Kansas, USA Journal of Herpetology PAUL BARTELT, Co-Editor Waldorf College Forest City, IA 50436, USA TIFFANY -

From Four Sites in Southern Amazonia, with A
Bol. Mus. Para. Emílio Goeldi. Cienc. Nat., Belém, v. 4, n. 2, p. 99-118, maio-ago. 2009 Squamata (Reptilia) from four sites in southern Amazonia, with a biogeographic analysis of Amazonian lizards Squamata (Reptilia) de quatro localidades da Amazônia meridional, com uma análise biogeográfica dos lagartos amazônicos Teresa Cristina Sauer Avila-PiresI Laurie Joseph VittII Shawn Scott SartoriusIII Peter Andrew ZaniIV Abstract: We studied the squamate fauna from four sites in southern Amazonia of Brazil. We also summarized data on lizard faunas for nine other well-studied areas in Amazonia to make pairwise comparisons among sites. The Biogeographic Similarity Coefficient for each pair of sites was calculated and plotted against the geographic distance between the sites. A Parsimony Analysis of Endemicity was performed comparing all sites. A total of 114 species has been recorded in the four studied sites, of which 45 are lizards, three amphisbaenians, and 66 snakes. The two sites between the Xingu and Madeira rivers were the poorest in number of species, those in western Amazonia, between the Madeira and Juruá Rivers, were the richest. Biogeographic analyses corroborated the existence of a well-defined separation between a western and an eastern lizard fauna. The western fauna contains two groups, which occupy respectively the areas of endemism known as Napo (west) and Inambari (southwest). Relationships among these western localities varied, except between the two northernmost localities, Iquitos and Santa Cecilia, which grouped together in all five area cladograms obtained. No variation existed in the area cladogram between eastern Amazonia sites. The easternmost localities grouped with Guianan localities, and they all grouped with localities more to the west, south of the Amazon River. -

Ecology of the Colubrid Snake Spilotes Pullatus from the Atlantic Forest of Southeastern Brazil Author(S): Otavio A
Ecology of the Colubrid Snake Spilotes pullatus from the Atlantic Forest of Southeastern Brazil Author(s): Otavio A. V. Marques, Diego F. Muniz-Da-Silva, Fausto E. Barbo, Silvia R. Travaglia Cardoso, Danusa C. Maia, and Selma M. Almeida-Santos Source: Herpetologica, 70(4):407-416. 2014. Published By: The Herpetologists' League DOI: http://dx.doi.org/10.1655/HERPETOLOGICA-D-14-00012 URL: http://www.bioone.org/doi/full/10.1655/HERPETOLOGICA-D-14-00012 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Herpetologica, 70(4), 2014, 407–416 Ó 2014 by The Herpetologists’ League, Inc. ECOLOGY OF THE COLUBRID SNAKE SPILOTES PULLATUS FROM THE ATLANTIC FOREST OF SOUTHEASTERN BRAZIL 1,5 2 3 OTAVIO A. V. MARQUES ,DIEGO F. MUNIZ-DA-SILVA ,FAUSTO E. BARBO ,SILVIA R. TRAVAGLIA 4 4 1 CARDOSO ,DANUSA C. -

Ecology of a Snake Assemblage in the Atlantic Forest of Southeastern Brazil
Volume 49(27):343‑360, 2009 Ecology of a snake assemblage in the Atlantic Forest of southeastern Brazil Paulo A. Hartmann1,3 Marília T. Hartmann1 Marcio Martins2 ABSTRACT The main objective of this study was to examine the natural history and the ecology of the species that constitute a snake assemblage in the Atlantic Rainforest, at Núcleo Picinguaba, Parque Estadual da Serra do Mar, located on the northern coast of the state of São Paulo, southeastern Brazil. The main aspects studied were: richness, relative abundance, daily and seasonal activity, and substrate use. We also provide additional information on natural history of the snakes. A total of 282 snakes, distributed over 24 species, belonging to 16 genera and four families, has been found within the area of the Núcleo Picinguaba. Species sampled more frequently were Bothrops jararaca and B. jararacussu. The methods that yielded the best results were time constrained search and opportunistic encounters. Among the abiotic factors analyzed, minimum temperature, followed by the mean temperature and the rainfall are apparently the most important in determining snake abundance. Most species presented a diet concentrated on one prey category or restricted to a few kinds of food items. The large number of species that feed on frogs points out the importance of this kind of prey as an important food resource for snakes in the Atlantic Rainforest. Our results indicate that the structure of the Picinguaba snake assemblage reflects mainly the phylogenetic constraints of each of its lineages Keywords: Assemblage; Snake; Diet; Habitat use; Activity. INTRODUCtiON species from the same lineage (taxocenes), and thus sharing at least part of their evolutionary history, One of the objectives of the study of commu- may provide valuable information about the different nity ecology is to identify patterns of resource use and ways by which distinct species respond to biotic and the mechanisms by which these patterns are achieved. -

Reptiles of Ecuador: a Resource-Rich Online Portal, with Dynamic
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [General Section]: 209–229 (e178). Reptiles of Ecuador: a resource-rich online portal, with dynamic checklists and photographic guides 1Omar Torres-Carvajal, 2Gustavo Pazmiño-Otamendi, and 3David Salazar-Valenzuela 1,2Museo de Zoología, Escuela de Ciencias Biológicas, Pontifcia Universidad Católica del Ecuador, Avenida 12 de Octubre y Roca, Apartado 17- 01-2184, Quito, ECUADOR 3Centro de Investigación de la Biodiversidad y Cambio Climático (BioCamb) e Ingeniería en Biodiversidad y Recursos Genéticos, Facultad de Ciencias de Medio Ambiente, Universidad Tecnológica Indoamérica, Machala y Sabanilla EC170301, Quito, ECUADOR Abstract.—With 477 species of non-avian reptiles within an area of 283,561 km2, Ecuador has the highest density of reptile species richness among megadiverse countries in the world. This richness is represented by 35 species of turtles, fve crocodilians, and 437 squamates including three amphisbaenians, 197 lizards, and 237 snakes. Of these, 45 species are endemic to the Galápagos Islands and 111 are mainland endemics. The high rate of species descriptions during recent decades, along with frequent taxonomic changes, has prevented printed checklists and books from maintaining a reasonably updated record of the species of reptiles from Ecuador. Here we present Reptiles del Ecuador (http://bioweb.bio/faunaweb/reptiliaweb), a free, resource-rich online portal with updated information on Ecuadorian reptiles. This interactive portal includes encyclopedic information on all species, multimedia presentations, distribution maps, habitat suitability models, and dynamic PDF guides. We also include an updated checklist with information on distribution, endemism, and conservation status, as well as a photographic guide to the reptiles from Ecuador. -

A Review of the Distribution of the Crested Eagle, Morphnus Guianensis (Daudin, 1800) (Accipitridae: Harpiinae), Including Range Extensions
Revista Brasileira de Ornitologia, 23(1), 36-63 March 2015 A review of the distribution of the Crested Eagle, Morphnus guianensis (Daudin, 1800) (Accipitridae: Harpiinae), including range extensions Felipe Bittioli R. Gomes1,3,4,5 and Tânia M. Sanaiotti2,3 1 Programa de Pós-Graduação em Ecologia – Instituto Nacional de Pesquisas da Amazônia – INPA. 2 Coordenação de Pesquisas em Biodiversidade - Instituto Nacional de Pesquisas da Amazônia – INPA 3 Programa de Conservação do Gavião-real (PCGR) – INPA 4 Current address: Laboratório de Zoologia, Programa de Pós-Graduação em Biodiversidade e Conservação, Universidade Federal do Pará – UFPA – Campus Universitário de Altamira. Rua José Porfírio, 2115, São Sebastião, CEP 68372-040, Altamira, PA, Brazil. 5 Corresponding author: [email protected] Received on 1 December 2014. Accepted on 22 January 2015. ABSTRACT: Here we review the distribution of the Crested Eagle (Morphnus guianensis) in the Americas, and based on the Brazilian Harpy Eagle Conservation Program (PCGR) database, literature, online databases, zoos, wild and museum records, we provide an updated distribution map with 37 points outside the IUCN map; 16 were recorded close to the border of the map (up to 40 km), and do not expand or contribute to the distribution map. Far from the border (>40 km) we found 21 records, contributing to an expansion of the known range and habitat. At the northernmost extreme of distribution, the range was extended to southern Mexico; in Nicaragua, the range extension was farther south in the north, and two records extend the range to the southern border with Costa Rica. In Colombia, an old specimen is located between Darien Peninsula and the Perija Mountains. -

Guyana 17 Sep-2 Oct 2016
Guyana 17 Sep-2 Oct 2016 Created by Roger Holmberg, Kungsör, Sweden (text, compilation, checklists, photo) [email protected] 1(84) Kaieteur Falls, one of the world’s highest free-falling waterfalls Diary This tour was booked with the local Guyana is bordered by the Atlantic Ocean company Ron Allicook Birding Tours. to the north, Brazil to the south-west and south-east, Suriname to the east and Guyana Venezuela to the north-west. With 215,000 What did I know about Guyana? As a square kilometres Guyana is the fourth- young kid I had glimpsed through some smallest country on mainland South nature books by adventurer Jan Lindblad, America. and became imposed by his photos. I’ll Most of Guyana's population, only 740,000 guess that’s when I initially got fascinated inhibitants, 90% lives in a narrow coastal by the rainforest. strip which makes up approximately 10% of the nation's total land area.The rest of the country is extremely low densitated. More than 80% of Guyana is still covered by pristine forests, from dry evergreen and seasonal forests to montane and lowland evergreen rain forests. There are hard to find another country with so many species of Cotingas, Macaws, Amazons and other Parrots. And the different pristine habitats hold a National flag of Guyana lots, lots of birds! 2(84) Top birds include Sun Parakeet, Red Siskin, Rufous-winged Ground-cuckoo, Guinean Cock-of-the-Rock, Crimson Fruitcrow, Capuchinbird, Crested Doradito (all new to me) and of course the Harpy Eagle. We start our trip by me going up to Stockholm Arlanda Airport, on 16 September, meeting up with Fredrik Rudzki for our first, short, leg. -

Diversity and Conservation Status of the Herpetofauna for an Area from North of Hidalgo, Mexico
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by SEDICI - Repositorio de la UNLP Trabajo Cuad. herpetol. 29 (2): 131-139 (2015) Diversity and conservation status of the herpetofauna for an area from north of Hidalgo, Mexico Luis M. Badillo-Saldaña, Aurelio Ramírez-Bautista, Daniel Lara-Tufiño, Christian Berriozabal-Islas Universidad Autónoma del Estado de Hidalgo. Centro de Investigaciones Biológicas. Laboratorio de Ecología de Poblaciones. Apartado Postal 1-69, Plaza Juárez, 42001 Pachuca, Hidalgo, México. Recibido: 27 Febrero 2015 ABSTRACT Revisado: 14 Mayo 2015 Conservation measures currently lack adequate information to assign some species of amphib- Aceptado: 09 Junio 2015 ians and reptiles in the categories of protection. In this study we analyzed and compared the Editor Asociado: M. Vaira herpetofauna of mountain cloud forest (MCF) and tropical evergreen forest (TEF) in an area north of Hidalgo. For this study, we conducted fieldwork (24 sites) and a literature review. In addition, the conservation status of species was analyzed. The herpetofauna of the municipal- ity of Tepehuacan de Guerrero, Hidalgo, Mexico consists of 70 species (20 amphibians and 50 reptiles), nine of which are historical records that were not found in the present study. Cloud forest was more diverse (39 species) than TEF (37 species). There are discrepancies between national and international agencies of conservation regarding the threatened status of these spe- cies. The high biodiversity recorded in MCF and TEF in the study area indicates the importance of this area for conservation. In this study, we propose to reassess the conservation category of Hidalgo state herpetofauna. -

Risk Assessment of Potential Invasiveness of Exotic Reptiles Imported to South Florida
Biol Invasions DOI 10.1007/s10530-009-9667-1 ORIGINAL PAPER Risk assessment of potential invasiveness of exotic reptiles imported to south Florida Ikuko Fujisaki • Kristen M. Hart • Frank J. Mazzotti • Kenneth G. Rice • Skip Snow • Michael Rochford Received: 5 February 2009 / Accepted: 16 November 2009 Ó Springer Science+Business Media B.V. 2009 Abstract The recent explosion of exotic reptiles in predict establishment success of 33 reptiles that were south Florida requires effective management strate- most frequently imported through Miami and St. gies. The objective of this study is to bring knowledge Petersburg ports from 2000 to 2005 and two additional of ecological correlates and quantitative modeling reptiles of concern in Florida, we identified eight methods into management by providing the foundation lizards and four snakes as potentially successful for a screening procedure that will identify potentially invaders. We further assessed adverse impacts associ- invasive species and assess adverse impacts associated ated with potential invaders, should they become with these species. We considered 17 variables and, established, by identifying species that are (1) danger- based on model selection procedures, we identified the ous to humans, (2) dangerous to the ecosystem (upper following significant predictors of establishment suc- trophic-level predators), and (3) rapidly spreading. cess: taxonomic order, maximum temperature match Controlling exotic reptiles can be expensive and labor between a species’ native range and Florida, animal intensive once they are established. Information on sale price, and manageability (defined as a species’ which species are potential invaders based on screen- maintenance cost, aggressiveness, proneness to ing procedures and what impacts these species might escape, and venomousness). -
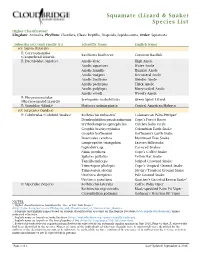
Squamate (Lizard & Snake) Species List
Squamate (Lizard & Snake) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Lepidosauria, Order: Squamata Suborder (sO:) and Family (F:) Scientific Name2 English Name2 sO: Sauria (Lizards) F: Corytophanidae Basiliscus basiliscus Common Basilisk (Casquehead Lizards) F: Dactyloidae (Anoles) Anolis altae High Anole Anolis aquaticus Water Anole Anolis humilis Humble Anole Anolis insignis Decorated Anole Anolis limifrons Slender Anole Anolis pachypus Thick Anole Anolis polylepis Many-scaled Anole Anolis woodi Wood's Anole F: Phrynosomatidae Sceloporus malachiticus Green Spiny Lizard (Phrynosomatid Lizards) F: Scincidae (Skinks) Marisora unimarginata Central American Mabuya sO: Serpentes (Snakes) F: Colubridae (Colubrid Snakes) Bothriechis nubestris5 Talamancan Palm-Pitviper5 Dendrophidion paucicarinatum Cope's Forest Racer Erythrolamprus epinephelus Culebra boba verde Geophis brachycephalus Colombian Earth Snake Geophis hoffmanni Hoffmann's Earth Snake Imantodes cenchoa Blunthead Tree Snake Lampropeltis triangulum Eastern Milksnake Leptodeira sp. Cat-eyed Snakes Ninia psephota Cope's Coffee Snake Spilotes pullatus Yellow Rat Snake Tantilla ruficeps Striped Crowned Snake3 Trimetopon pliolepis Cope's Tropical Ground Snake Trimetopon slevini Slevin's Tropical Ground Snake Urotheca decipiens Pale Ground Snake Urotheca guentheri Gunther's Graceful Brown Snake4 F: Viperidae (Vipers) Bothriechis lateralis Coffee Palm Viper Bothriechis nigroviridis5 Black-speckled Palm Pit Viper5 Cerrophidion godmani Godman's Montane Pit Viper NOTES: 1, Higher classification as found on the Tree of Life Web Project (http://tolweb.org/accessory/Phylogeny_and_Classification_of_Amniotes?acc_id=462). 2, Scientific and English names based on current classifications as found on The Reptile Database (www.reptile-database.org), unless indicated otherwise. 3, English name as found on SnakeDatabase (http://snakedatabase.org/species/tantilla/ruficeps). 4, English name as found on The Encyclopedia of Life (http://eol.org/pages/1055095/overview).