CHIMPANZEE (Pan Troglodytes) CARE MANUAL
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

EAZA Best Practice Guidelines Bonobo (Pan Paniscus)
EAZA Best Practice Guidelines Bonobo (Pan paniscus) Editors: Dr Jeroen Stevens Contact information: Royal Zoological Society of Antwerp – K. Astridplein 26 – B 2018 Antwerp, Belgium Email: [email protected] Name of TAG: Great Ape TAG TAG Chair: Dr. María Teresa Abelló Poveda – Barcelona Zoo [email protected] Edition: First edition - 2020 1 2 EAZA Best Practice Guidelines disclaimer Copyright (February 2020) by EAZA Executive Office, Amsterdam. All rights reserved. No part of this publication may be reproduced in hard copy, machine-readable or other forms without advance written permission from the European Association of Zoos and Aquaria (EAZA). Members of the European Association of Zoos and Aquaria (EAZA) may copy this information for their own use as needed. The information contained in these EAZA Best Practice Guidelines has been obtained from numerous sources believed to be reliable. EAZA and the EAZA APE TAG make a diligent effort to provide a complete and accurate representation of the data in its reports, publications, and services. However, EAZA does not guarantee the accuracy, adequacy, or completeness of any information. EAZA disclaims all liability for errors or omissions that may exist and shall not be liable for any incidental, consequential, or other damages (whether resulting from negligence or otherwise) including, without limitation, exemplary damages or lost profits arising out of or in connection with the use of this publication. Because the technical information provided in the EAZA Best Practice Guidelines can easily be misread or misinterpreted unless properly analysed, EAZA strongly recommends that users of this information consult with the editors in all matters related to data analysis and interpretation. -

Gorilla Beringei (Eastern Gorilla) 07/09/2016, 02:26
Gorilla beringei (Eastern Gorilla) 07/09/2016, 02:26 Kingdom Phylum Class Order Family Animalia ChordataMammaliaPrimatesHominidae Scientific Gorilla beringei Name: Species Matschie, 1903 Authority: Infra- specific See Gorilla beringei ssp. beringei Taxa See Gorilla beringei ssp. graueri Assessed: Common Name(s): English –Eastern Gorilla French –Gorille de l'Est Spanish–Gorilla Oriental TaxonomicMittermeier, R.A., Rylands, A.B. and Wilson D.E. 2013. Handbook of the Mammals of the World: Volume Source(s): 3 Primates. Lynx Edicions, Barcelona. This species appeared in the 1996 Red List as a subspecies of Gorilla gorilla. Since 2001, the Eastern Taxonomic Gorilla has been considered a separate species (Gorilla beringei) with two subspecies: Grauer’s Gorilla Notes: (Gorilla beringei graueri) and the Mountain Gorilla (Gorilla beringei beringei) following Groves (2001). Assessment Information [top] Red List Category & Criteria: Critically Endangered A4bcd ver 3.1 Year Published: 2016 Date Assessed: 2016-04-01 Assessor(s): Plumptre, A., Robbins, M. & Williamson, E.A. Reviewer(s): Mittermeier, R.A. & Rylands, A.B. Contributor(s): Butynski, T.M. & Gray, M. Justification: Eastern Gorillas (Gorilla beringei) live in the mountainous forests of eastern Democratic Republic of Congo, northwest Rwanda and southwest Uganda. This region was the epicentre of Africa's "world war", to which Gorillas have also fallen victim. The Mountain Gorilla subspecies (Gorilla beringei beringei), has been listed as Critically Endangered since 1996. Although a drastic reduction of the Grauer’s Gorilla subspecies (Gorilla beringei graueri), has long been suspected, quantitative evidence of the decline has been lacking (Robbins and Williamson 2008). During the past 20 years, Grauer’s Gorillas have been severely affected by human activities, most notably poaching for bushmeat associated with artisanal mining camps and for commercial trade (Plumptre et al. -

2017 Courses at Wellesley and Workshops
EXPLORE THE WORLD OF PEOPLE AND IDEAS Zombinomics Pandemic! Outbreak! Go! Post-Apocalyptic Economics Bacterial Epidemiology E-20 Summit Fix It + Flip It Crime Squad So, You Want to Be a Doctor? World Economics Interior Remodeling Design Criminal Investigations Medical Careers THIS GUIDE IS FOR REFERENCE ONLY Cupcake Armada Processing Makes Perfect Perfect Pixels Flying Ninjas Cupcake ChallengesThe coursesComputer and Programming workshops listedIntro toin Digital this Photography guide were offeredAerial Robotics during the summer of 2017. While we will offer a similar range of courses and workshops in 2018, specific options may change. 2018 options will be published later in the fall. Sing It? Bring It! The Wig + the Wardrobe Pigskin Payoff Accessorize This! Pop Choir Music Costume, Hair + Make-up Design Business of Sports Intro to Accessory Design Sky Scraping Race on Mars! Manufacturing Mario Cooks Unhooked Commercial Architecture Exploratory Robotics Conceptual Video Game Design Cooking Challenges 2017 Courses and Workshops at Wellesley EXPLO 360 at Wellesley: Courses Summer 2017 EXPLORE THE WORLD OF PEOPLE AND IDEAS… AND DISCOVER YOUR PLACE IN IT Step out of your comfort zone and explore the world of people and ideas. Whether you’re an expert in algebra, basketball, or ceramics, – or whether you’ve only imagined being a fashion Curriculum Innovation designer, a sports agent, or a crime scene scientist, – EXPLO lets at EXPLO you dive into what you love as well as what you dream about. The choice is all yours. Our curriculum team spends 10 months a year The courses and workshops at EXPLO 360 at Wellesley are your planning and preparing opportunity to develop and express your natural talents or explore new the hundreds of courses interests and skills in a pressure-free environment (That’s right — and workshops that EXPLO no grades!) with other students offers each summer. -

Bonobos (Pan Paniscus) Show an Attentional Bias Toward Conspecifics’ Emotions
Bonobos (Pan paniscus) show an attentional bias toward conspecifics’ emotions Mariska E. Kreta,1, Linda Jaasmab, Thomas Biondac, and Jasper G. Wijnend aInstitute of Psychology, Cognitive Psychology Unit, Leiden University, 2333 AK Leiden, The Netherlands; bLeiden Institute for Brain and Cognition, 2300 RC Leiden, The Netherlands; cApenheul Primate Park, 7313 HK Apeldoorn, The Netherlands; and dPsychology Department, University of Amsterdam, 1018 XA Amsterdam, The Netherlands Edited by Susan T. Fiske, Princeton University, Princeton, NJ, and approved February 2, 2016 (received for review November 8, 2015) In social animals, the fast detection of group members’ emotional perspective, it is most adaptive to be able to quickly attend to rel- expressions promotes swift and adequate responses, which is cru- evant stimuli, whether those are threats in the environment or an cial for the maintenance of social bonds and ultimately for group affiliative signal from an individual who could provide support and survival. The dot-probe task is a well-established paradigm in psy- care (24, 25). chology, measuring emotional attention through reaction times. Most primates spend their lives in social groups. To prevent Humans tend to be biased toward emotional images, especially conflicts, they keep close track of others’ behaviors, emotions, and when the emotion is of a threatening nature. Bonobos have rich, social debts. For example, chimpanzees remember who groomed social emotional lives and are known for their soft and friendly char- whom for long periods of time (26). In the chimpanzee, but also in acter. In the present study, we investigated (i) whether bonobos, the rarely studied bonobo, grooming is a major social activity and similar to humans, have an attentional bias toward emotional scenes a means by which animals living in proximity may bond and re- ii compared with conspecifics showing a neutral expression, and ( ) inforce social structures. -

Orangutan…Taxonomy…And…Nomenclature
«««« ORANGUTAN…TAXONOMY…AND…NOMENCLATURE« « Craig«D em itros« « The«taxonom y«of«the«orangutan«has«been«confusing«and«is«still«the«subject«of« m uch«debate.«Q uestions«at«the«specific«and«subspecific«level«are«still«being« investigated«(Courtenay«et«al.«1988).«The«follow ing«taxonom ic«inform ation«is« taken«prim arily«from «G roves,«1971.« « H IG H ER«LEVEL«TAXO N O M Y:« O rder:«Prim ates« Suborder:«A nthropoidea« Superfam ily:«H om inoidea« Fam ily:«Pongidae«(Includes«extant«genera«Pan,…Gorilla…and…Pongo).« « H ISTO RICA L«TAXO N O M Y«AT«TH E«G EN U S«A N D «G EN U S«SPECIES«LEVEL:«« G enus« Pongo«Lacepede,«1799.« O urangus«Zim m erm an,«1777«(N am e«invalidated).« « G enus«species«(Pongo…pygm aeus«H oppius,«1763).« Sim ia…pygm aeus«H oppius,«1763.««Type«locality«Sum atra.« Sim ia…satyrus«Linnaeus,«1766.« O urangus…outangus«Zim m erm an,«1777.« Pongo…borneo«Lacepede,«1799.««Type«locality«Borneo.« Sim ia…Agrais«Schreber,«1779.««Type«locality«Borneo.« Pongo…W urm bii«Tiedem ann,«1808.««Type«locality«Borneo.« Pongo…Abelii«Lesson,«1827.««Type«locality«Sum atra.« Sim ia…M orio«O w en,«1836.««Type«locality«Borneo.« Pithecus…bicolor«I.«G eoffroy,«1841.««Type«locality«Sum atra.« Sim ia…Gargantica«Pearson,«1841.««Type«locality«Sum atra.« Pithecus…brookei«Blyth,«1853.««Type«locality«Saraw ak.« Pithecus…ow enii«Blyth,«1853.««Type«locality«Saraw ak.« Pithecus…curtus«Blyth,«1855.««Type«locality«Saraw ak.« Satyrus…Knekias«M eyer,«1856.««Type«locality«Borneo.« Pithecus…W allichii«G ray,«1870.««Type«locality«Borneo.« Pithecus…sum atranus«Selenka,«1896.««Type«locality«Sum atra.« Pongo…pygm aeus«Rothschild,«1904.««First«use«of«this«com bination.« Ptihecus…w allacei«Elliot,«1913.««Type«locality«Borneo.« « CURRENT…TAXONOMY« « The«current«and«m ost«accepted«taxonom y«of«the«G enus«Pongo«includes«one« species«Pongo…pygm aeus«and«tw o«subspecies«P.p.…pygm aeus«(the«Bornean« subspecies)«and«P.p.…abelii«(the«Sum atran«subspecies)«(Bem m el«1968;«Jones« 1969;«G roves«1971;«Jacobshagen«1979;«Seuarez«et«al.«1979«and«G roves«1993).« 5« « . -

Cross-River Gorillas
CMS Agreement on the Distribution: General Conservation of Gorillas UNEP/GA/MOP3/Inf.9 and their Habitats of the 24 April 2019 Convention on Migratory Species Original: English THIRD MEETING OF THE PARTIES Entebbe, Uganda, 18-20 June 2019 REVISED REGIONAL ACTION PLAN FOR THE CONSERVATION OF THE CROSS RIVER GORILLA (Gorilla gorilla diehli) 2014 - 2019 For reasons of economy, this document is printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. FewerToday, thethan total population of Cross River gorillas may number fewer than 300 individuals 300 left Revised Regional Action Plan for the Conservation of the Cross River Gorilla (Gorilla gorilla diehli) 2014–2019 HopeUnderstanding the status of the changing threats across the Cross River gorilla landscape will provide key information for guiding our collectiveSurvival conservation activities cross river gorilla action plan cover_2013.indd 1 2/3/14 10:27 AM Camera trap image of a Cross River gorilla at Afi Mountain Cross River Gorilla (Gorilla gorilla diehli) This plan outlines measures that should ensure that Cross River gorilla numbers are able to increase at key core sites, allowing them to extend into areas where they have been absent for many years. cross river gorilla action plan cover_2013.indd 2 2/3/14 10:27 AM Revised Regional Action Plan for the Conservation of the Cross River Gorilla (Gorilla gorilla diehli) 2014-2019 Revised Regional Action Plan for the Conservation of the Cross River Gorilla (Gorilla gorilla diehli) 2014-2019 Compiled and edited by Andrew Dunn1, 16, Richard Bergl2, 16, Dirck Byler3, Samuel Eben-Ebai4, Denis Ndeloh Etiendem5, Roger Fotso6, Romanus Ikfuingei6, Inaoyom Imong1, 7, 16, Chris Jameson6, Liz Macfie8, 16, Bethan Mor- gan9, 16, Anthony Nchanji6, Aaron Nicholas10, Louis Nkembi11, Fidelis Omeni12, John Oates13, 16, Amy Pokemp- ner14, Sarah Sawyer15 and Elizabeth A. -

Primates Don't Make Good Pets! Says Lincoln Park
FOR IMMEDIATE RELEASE EDITOR’S NOTE: Photos of an appropriate multi-male, multi-female group of chimpanzees at Lincoln Park Zoo can be found HERE. Pet trade images are not shared as Lincoln Park Zoo research shows when these images of chimpanzees in human settings are circulated, chimpanzees are not believed to be endangered. Primates Don’t Make Good Pets! Says Lincoln Park Zoo Series of manuscripts from the Lester E. Fisher Center for the Study and Conservation of Apes bring light to the detrimental effects of atypically-housed chimpanzees Chicago (December 13, 2017) – The Wolf of Wall Street movie. Weezer’s “Island in the Sun” music video. Michael Jackson’s “pet” Bubbles. While these may seem like unrelated pop culture references, they all have a similarly daunting theme: the use of chimpanzees in the pet or entertainment trade. These chimpanzees typically are raised by humans and rarely see others of their own species until they are fortunate enough to be moved to an accredited zoo or sanctuary. For years, Lincoln Park Zoo researchers have documented the long-term effects of this unusual human exposure on chimpanzees. Now, a third and final study in a series has been published in Royal Society Open Science Dec. 13 showcasing the high stress levels experienced by these chimpanzees who have been raised in human homes and trained to perform for amusement. Over the course of the three years, Fisher Center researchers evaluated more than 60 chimpanzees – all now living in accredited zoos and sanctuaries - and examined the degree to which they were exposed to humans and to their own species over their lifetime to determine the long-term effects of such exposure. -

Proposal for Inclusion of the Chimpanzee
CMS Distribution: General CONVENTION ON MIGRATORY UNEP/CMS/COP12/Doc.25.1.1 25 May 2017 SPECIES Original: English 12th MEETING OF THE CONFERENCE OF THE PARTIES Manila, Philippines, 23 - 28 October 2017 Agenda Item 25.1 PROPOSAL FOR THE INCLUSION OF THE CHIMPANZEE (Pan troglodytes) ON APPENDIX I AND II OF THE CONVENTION Summary: The Governments of Congo and the United Republic of Tanzania have jointly submitted the attached proposal* for the inclusion of the Chimpanzee (Pan troglodytes) on Appendix I and II of CMS. *The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CMS Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. UNEP/CMS/COP12/Doc.25.1.1 PROPOSAL FOR THE INCLUSION OF CHIMPANZEE (Pan troglodytes) ON APPENDICES I AND II OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A: PROPOSAL Inclusion of Pan troglodytes in Appendix I and II of the Convention on the Conservation of Migratory Species of Wild Animals. B: PROPONENTS: Congo and the United Republic of Tanzania C: SUPPORTING STATEMENT 1. Taxonomy 1.1 Class: Mammalia 1.2 Order: Primates 1.3 Family: Hominidae 1.4 Genus, species or subspecies, including author and year: Pan troglodytes (Blumenbach 1775) (Wilson & Reeder 2005) [Note: Pan troglodytes is understood in the sense of Wilson and Reeder (2005), the current reference for terrestrial mammals used by CMS). -

Michael Jackson Betwixt and Between: the Construction of Identity in 'Leave Me Alone' (1989)
Contents Introduction 2 Chapter 1: Literature Review 4 1.1: Past Research: On Michael Jackson Studies 4 1.2: Disability Studies 4 1.2.1: The Static Freak/Plastic Freak 6 1.3: Defining the Liminal 7 1.4: Postmodern Theory: The Social Construction of Identity 8 Chapter 2: Methodology 10 Chapter 3: Analysis 12 3.1: Narrative Structure of ‘Leave Me Alone’ 12 3.2: Media Narratives in ‘Leave Me Alone’ 13 3.3: The Static Freak/Plastic Freak in ‘Leave Me Alone’ 15 3.4: Liminal Identity: Temporary Phase/Permanent Place 16 3.5: Postmodern Subjects: The Aesthetics of the Collage 17 3.6: Identity and Postmodernism: The Problem With Disability Studies 18 Chapter 4: Conclusion 20 Bibliography 22 Attachment 1: Images 24 Attachment 2: Shotlist ‘Leave Me Alone’ 27 1 Introduction ‘The bottom line is they don’t know and everyone is going to continue searching to find out whether I’m gay, straight, or whatever … And the longer it takes to discover this, the more famous I will be.’1 * * * ‘Michael’s space-age diet’, ‘Bubbles the chimp bares all about Michael’, ‘Michael proposes to Liz’, ‘Michael to marry Brooke’. These may look like part of the usual rumors about Jackson frequently appearing in the media, however, they are not. Instead, these headlines are the opening scene of the music video for Jackson’s song ‘Leave Me Alone’, released on January 2, 1989, and directed by Jim Blashfield. Jackson opens by singing the words: I don't care what you talkin' 'bout baby I don't care what you say Don't you come walkin' beggin' back mama I don't care anyway ‘Leave Me Alone’ is a response to the rumors that began to circulate in the media after the worldwide success of Jackson’s 1982 album Thriller. -
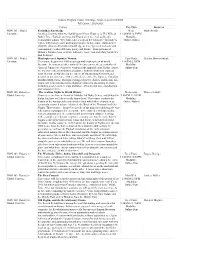
Honors Curriculum Sheet
Honors Program Course Offerings, Updated as of 8/14/2020 Fall Quarter, 2020-2021 Course Day/Time Instructor HON 101 - World Forbidden Knowledge Wed: Mark Arendt Literature Are there limits to what we should know? From Chaucer, in The Wife of 11:20AM-12:50PM Bath’s Tale, “Forbede us thing and That desiren we,” to Lou Reed’s Modality: Transformer album, “Hey babe, take a walk on the wild side,” literature is Online-Hybrid replete with transgressors and transgressions. In this course students will study the subject of forbidden knowledge as it is expressed in classic and contemporary works of fiction, poetry and drama – from portions of Milton’s Paradise Lost to Denis Johnson’s Jesus’ Son and Mary Gaitskill’s Bad Behavior. HON 101 - World Masterpieces of Japanese Women Tues/Thurs: Heather Bowen-Struyk Literature This course begins over 1000 years ago with masterpieces of world 1:00PM-2:30PM literature. In contrast to other national literary canons, the great works of Modality: classical Japan were written by women in the imperial court. In this course, Online-Sync we will travel the socio-historical distance from the women of classical court literature to Raichō and her coterie of bluestocking feminists and beyond, to our own time, with a self-reflexive novel by Japanese-Canadian Buddhist Ruth Ozeki. Through readings of poetry, diaries, and fiction, this course offers an introduction to important issues for discussing literature including gender and sex, class and labor, ethnicity and race, and diaspora and national identity. HON 102: History in The Arabian Nights in World History Wednesday: Warren Schultz Global Contexts Chances are we have all heard of Aladdin, Ali Baba, Genies, and Sinbad the 9:40AM-11:10AM Sailor, but how well do we really know them? This course explores the Modality: history of the famous collection of tales from which these characters are Online-Hybrid commonly assumed to have inhabited, the Book of the Thousand and One Nights. -

98.6: a Creative Commonality
CONTENT 1 - 2 exibition statement 3 - 18 about the chimpanzees and orangutans 19 resources 20 educational activity 21-22 behind the scenes 23 installation images 24 walkthrough video / flickr page 25-27 works in show 28 thank you EXHIBITION STATEMENT Humans and chimpanzees share 98.6% of the same DNA. Both species have forward-facing eyes, opposing thumbs that accompany grasping fingers, and the ability to walk upright. Far greater than just the physical similarities, both species have large brains capable of exhibiting great intelligence as well as an incredible emotional range. Chimpanzees form tight social bonds, especially between mothers and children, create tools to assist with eating and express joy by hugging and kissing one another. Over 1,000,000 chimpanzees roamed the tropical rain forests of Africa just a century ago. Now listed as endangered, less than 300,000 exist in the wild because of poaching, the illegal pet trade and habitat loss due to human encroachment. Often, chimpanzees are killed, leaving orphans that are traded and sold around the world. Thanks to accredited zoos and sanctuaries across the globe, strong conservation efforts and programs exist to protect and manage populations of many species of the animal kingdom, including the great apes - the chimpanzee, gorilla, orangutan and bonobo. In the United States, institutions such as the Association of Zoos and Aquariums (AZA) and the Species Survival Plan (SSP) work together across the nation in a cooperative effort to promote population growth and ensure the utmost care and conditions for all species. Included in the daily programs for many species is what’s commonly known as “enrichment”–– an activity created and employed to stimulate and pose a challenge, such as hiding food and treats throughout an enclosure that requires a search for food, sometimes with a problem-solving component. -

West African Chimpanzees
Status Survey and Conservation Action Plan West African Chimpanzees Compiled and edited by Rebecca Kormos, Christophe Boesch, Mohamed I. Bakarr and Thomas M. Butynski IUCN/SSC Primate Specialist Group IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and West African Chimpanzees Action Plan The IUCN Species Survival Commission is committed to communicating important species conservation information to natural resource managers, decision makers and others whose actions affect the conservation of biodiversity. The SSC’s Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC’s Wildlife Trade Programme and Conser- vation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conser- vation of natural landscapes, coordination of law enforcement efforts, as well as promotion of conservation education, research, and international cooperation. The World Wide Fund for Nature (WWF) provides significant annual operating support to the SSC.