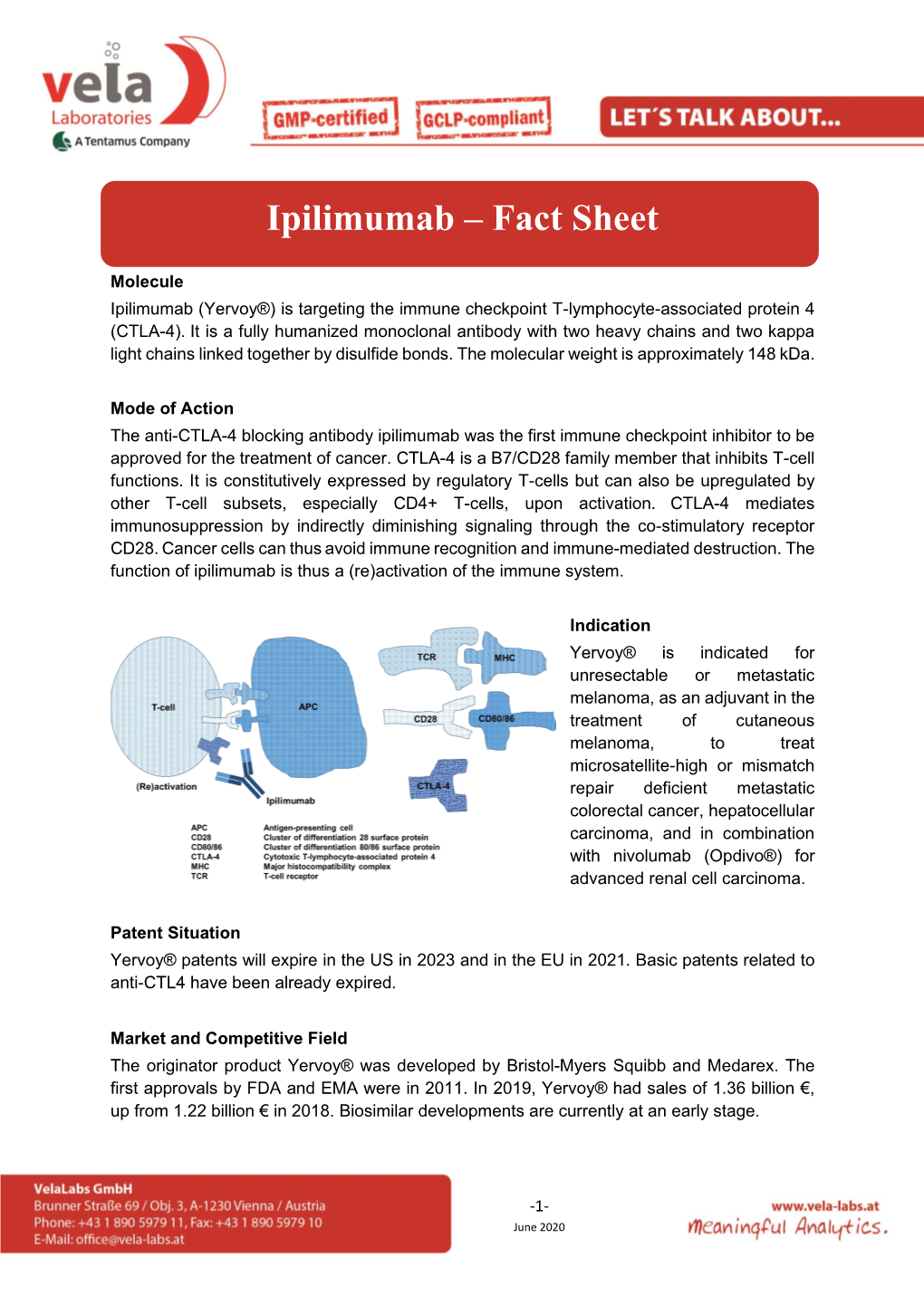
Ipilimumab – Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Anti-CTLA4 Antibody) Causes Regression of Metastatic Renal Cell Cancer Associated with Enteritis and Hypophysitis James C
CLINICAL STUDY Ipilimumab (Anti-CTLA4 Antibody) Causes Regression of Metastatic Renal Cell Cancer Associated With Enteritis and Hypophysitis James C. Yang,* Marybeth Hughes,* Udai Kammula,* Richard Royal,* Richard M. Sherry,* Suzanne L. Topalian,* Kimberly B. Suri,* Catherine Levy,* Tamika Allen,* Sharon Mavroukakis,* Israel Lowy,w Donald E. White,* and Steven A. Rosenberg* (J Immunother 2007;30:825–830) Summary: The inhibitory receptor CTLA4 has a key role in peripheral tolerance of T cells for both normal and tumor- associated antigens. Murine experiments suggested that block- ade of CTLA4 might have antitumor activity and a clinical t has become clear that T-cell responses are under the experience with the blocking antibody ipilimumab in patients Icontrol of both activating and inhibitory coreceptors.1,2 with metastatic melanoma did show durable tumor regressions The interaction of the T-cell receptor with its cognate in some patients. Therefore, a phase II study of ipilimumab was epitope, presented by the correct major histocompatibility conducted in patients with metastatic renal cell cancer with a complex molecule (‘‘signal one’’) is only one component primary end point of response by Response Evaluation Criteria of the complex signaling required to optimally activate a in Solid Tumors (RECIST) criteria. Two sequential cohorts T cell to proliferate, differentiate, and exhibit effector received either 3 mg/kg followed by 1 mg/kg or all doses at functions.3 The critical role of inhibitory signaling in 3 mg/kg every 3 weeks (with no intention of comparing cohort preventing, limiting, or terminating T-cell responses has response rates). Major toxicities were enteritis and endocrine recently become apparent.4 T-regulatory cells (T-regs) deficiencies of presumed autoimmune origin. -

BAVENCIO (Avelumab) Injection, for Intravenous Use Function
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------WARNINGS AND PRECAUTIONS---------------------- These highlights do not include all the information needed to use • Immune-mediated pneumonitis: Withhold for moderate pneumonitis; BAVENCIO safely and effectively. See full prescribing information for permanently discontinue for severe, life-threatening, or recurrent moderate BAVENCIO. pneumonitis. (5.1) • Hepatotoxicity and immune-mediated hepatitis: Monitor for changes in liver ® BAVENCIO (avelumab) injection, for intravenous use function. Withhold for moderate hepatitis; permanently discontinue for Initial U.S. Approval: 2017 severe or life-threatening hepatitis. (5.2) • Immune-mediated colitis: Withhold for moderate or severe colitis; ----------------------------RECENT MAJOR CHANGES-------------------------- permanently discontinue for life-threatening or recurrent severe colitis. (5.3) Indication and Usage (1.3) 05/2019 • Immune-mediated endocrinopathies: Withhold for severe or life-threatening Dosage and Administration (2.2, 2.3) 10/2018 endocrinopathies (5.4) Dosage and Administration (2.4, 2.5) 05/2019 • Immune-mediated nephritis and renal dysfunction: Withhold for moderate Warnings and Precautions (5.2, 5.8) 05/2019 or severe nephritis and renal dysfunction; permanently discontinue for life- threatening nephritis or renal dysfunction. (5.5) ----------------------------INDICATIONS AND USAGE-------------------------- • Infusion-related reactions: Interrupt or slow the rate of infusion for mild or BAVENCIO is a programmed death ligand-1 (PD-L1) blocking antibody moderate infusion-related reactions. Stop the infusion and permanently indicated for: discontinue BAVENCIO for severe or life-threatening infusion-related • Adults and pediatric patients 12 years and older with metastatic Merkel cell a reactions. (5.7) carcinoma (MCC). (1.1) • Major adverse cardiovascular events: Optimize management of • Patients with locally advanced or metastatic urothelial carcinoma (UC) cardiovascular risk factors. -

Reference ID: 4734770 Dexamethasone Is Not Recommended Outside of Controlled Clinical Trials
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------DOSAGE AND ADMINISTRATION------------------- These highlights do not include all the information needed to use OPDIVO • Administer by intravenous infusion based upon recommended infusion rate safely and effectively. See full prescribing information for OPDIVO. for each indication. (2) OPDIVO (nivolumab) injection, for intravenous use • Unresectable or metastatic melanoma Initial U.S. Approval: 2014 • 240 mg every 2 weeks or 480 mg every 4 weeks. (2.2) • 1 mg/kg followed by ipilimumab 3 mg/kg on the same day every 3 weeks --------------------------RECENT MAJOR CHANGES--------------------------- for 4 doses, then 240 mg every 2 weeks or 480 mg every 4 weeks. (2.2) Indications and Usage (1) 1/2021 • Adjuvant treatment of melanoma Dosage and Administration (2) 1/2021 • 240 mg every 2 weeks or 480 mg every 4 weeks. (2.2) Warnings and Precautions (5) 1/2021 • Metastatic non-small cell lung cancer ---------------------------INDICATIONS AND USAGE--------------------------- • 3 mg/kg every 2 weeks with ipilimumab 1 mg/kg every 6 weeks. (2.2) OPDIVO is a programmed death receptor-1 (PD-1) blocking antibody indicated • 360 mg every 3 weeks with ipilimumab 1 mg/kg every 6 weeks and for the treatment of: 2 cycles of platinum-doublet chemotherapy. (2.2) Melanoma • 240 mg every 2 weeks or 480 mg every 4 weeks. (2.2) • patients with unresectable or metastatic melanoma, as a single agent or in • Malignant pleural mesothelioma combination with ipilimumab. (1.1) • 360 mg every 3 weeks with ipilimumab 1 mg/kg every 6 weeks. (2.2) • patients with melanoma with lymph node involvement or metastatic disease • Advanced renal cell carcinoma who have undergone complete resection, in the adjuvant setting. -

Immune-Checkpoint Inhibitors from Cancer to COVID-19
INTERNATIONAL JOURNAL OF ONCOLOGY 58: 145-157, 2021 Immune-checkpoint inhibitors from cancer to COVID-19: A promising avenue for the treatment of patients with COVID-19 (Review) SILVIA VIVARELLI1, LUCA FALZONE2, FRANCESCO TORINO3, GIUSEPPA SCANDURRA4, GIULIA RUSSO5, ROBERTO BORDONARO6, FRANCESCO PAPPALARDO5,7, DEMETRIOS A. SPANDIDOS8, GIUSEPPINA RACITI5* and MASSIMO LIBRA1,7* 1Section of General Pathology, Clinics and Oncology, Department of Biomedical and Biotechnological Sciences, University of Catania, I-95123 Catania; 2Epidemiology Unit, IRCCS Istituto Nazionale Tumori ‘Fondazione G. Pascale’, I-80131 Naples; 3Department of Systems Medicine, Medical Oncology, University of Rome Tor Vergata, I-00133 Rome; 4Medical Oncology Unit, Azienda Ospedaliera Cannizzaro, I-95126 Catania; 5Department of Drug Sciences, University of Catania, I-95123 Catania; 6Medical Oncology Unit, Garibaldi Hospital, I-95122 Catania; 7Research Center for Prevention, Diagnosis and Treatment of Tumors, University of Catania, I-95123 Catania, Italy; 8Laboratory of Clinical Virology, Medical School, University of Crete, 71003 Heraklion, Greece Received November 5, 2020; Accepted December 14, 2020 DOI: 10.3892/ijo.2020.5159 Abstract. The severe acute respiratory syndrome associ- suffer from T-cell exhaustion, which may lead to viral sepsis ated coronavirus-2 (SARS-CoV-2) poses a threat to human and an increased mortality rate. It has been observed that cancer life worldwide. Since early March, 2020, coronavirus patients, who usually are immunocompromised, may restore disease 2019 (COVID-19), characterized by an acute and often their anti-tumoral immune response when treated with ICIs. severe form of pneumonia, has been declared a pandemic. Moreover, viral-infected mice and humans, exhibit a T-cell This has led to a boom in biomedical research studies at all exhaustion, which is also observed following SARS-CoV-2 stages of the pipeline, from the in vitro to the clinical phase. -

S41467-019-11980-6.Pdf
ARTICLE https://doi.org/10.1038/s41467-019-11980-6 OPEN Potent antibody lineage against malaria transmission elicited by human vaccination with Pfs25 Brandon McLeod1,2, Kazutoyo Miura 3, Stephen W. Scally1, Alexandre Bosch1, Ngan Nguyen4, Hanjun Shin4, Dongkyoon Kim4, Wayne Volkmuth4, Sebastian Rämisch 5, Jessica A. Chichester6, Stephen Streatfield7, Colleen Woods8, William R. Schief 5, Daniel Emerling4, C. Richter King 8 & Jean-Philippe Julien 1,2,9 1234567890():,; Transmission-blocking vaccines have the potential to be key contributors to malaria elim- ination. Such vaccines elicit antibodies that inhibit parasites during their development in Anopheles mosquitoes, thus breaking the cycle of transmission. To date, characterization of humoral responses to Plasmodium falciparum transmission-blocking vaccine candidate Pfs25 has largely been conducted in pre-clinical models. Here, we present molecular analyses of human antibody responses generated in a clinical trial evaluating Pfs25 vaccination. From a collection of monoclonal antibodies with transmission-blocking activity, we identify the most potent transmission-blocking antibody yet described against Pfs25; 2544. The interactions of 2544 and three other antibodies with Pfs25 are analyzed by crystallography to understand structural requirements for elicitation of human transmission-blocking responses. Our ana- lyses provide insights into Pfs25 immunogenicity and epitope potency, and detail an affinity maturation pathway for a potent transmission-blocking antibody in humans. Our findings can be employed to guide the design of improved malaria transmission-blocking vaccines. 1 Program in Molecular Medicine, The Hospital for Sick Children Research Institute, 686 Bay Street, Toronto, ON M5G 0A4, Canada. 2 Department of Biochemistry, University of Toronto, 1 King’s College Circle, Toronto, ON M5S 1A8, Canada. -

Inhibiting Phagocytosis with Cd47: from the Effects of Red Cell Rigidity and Shape to Display on Lentivirus - Implications for Aging and Gene Therapy
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2014 Inhibiting Phagocytosis With Cd47: From the Effects of Red Cell Rigidity and Shape to Display on Lentivirus - Implications for Aging and Gene Therapy Nisha Gowri Sosale University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Biophysics Commons, Chemical Engineering Commons, and the Virology Commons Recommended Citation Sosale, Nisha Gowri, "Inhibiting Phagocytosis With Cd47: From the Effects of Red Cell Rigidity and Shape to Display on Lentivirus - Implications for Aging and Gene Therapy" (2014). Publicly Accessible Penn Dissertations. 1451. https://repository.upenn.edu/edissertations/1451 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/1451 For more information, please contact [email protected]. Inhibiting Phagocytosis With Cd47: From the Effects of Red Cell Rigidity and Shape to Display on Lentivirus - Implications for Aging and Gene Therapy Abstract A macrophage engulfs another cell, or foreign particle, via phagocytosis, an engulfment process crucial not only to innate and adaptive immunity, but also to the maintenance of homeostasis. Phagocytosis is a receptor-mediated process that is dependent on Myosin-IIA motors, among other cytoskeletal proteins. Adhesion processes of both hematopoietic and mesenchymal derived cells can activate Myosin, and increasingly so on rigid substrates. Macrophage engulfment becomes inefficient if the macrophage also engages `Marker of Self' CD47 that inhibits Myosin accumulation to the phagocytic synapse. CD47 is a ubiquitously expressed transmembrane cell surface protein that binds to signal regulatory protein alpha (SIRPA) that is highly expressed by macrophages. CD47's role in downregulating macrophage phagocytosis was first discovered in murine erythrocytes (RBCs), where wild-type RBCs are long-lived in circulation, while RBCs derived from a CD47 knockout mouse are rapidly cleared. -

A Reappraisal of CTLA-4 Checkpoint Blockade in Cancer Immunotherapy
www.nature.com/cr ARTICLE OPEN A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy Xuexiang Du1, Fei Tang1, Mingyue Liu1, Juanjuan Su1, Yan Zhang1, Wei Wu1, Martin Devenport2, Christopher A Lazarski1, Peng Zhang1, Xu Wang1, Peiying Ye1, Changyu Wang3, Eugene Hwang1, Tinghui Zhu4, Ting Xu4, Pan Zheng1,2 and Yang Liu1,2 It is assumed that anti-CTLA-4 antibodies cause tumor rejection by blocking negative signaling from B7-CTLA-4 interactions. Surprisingly, at concentrations considerably higher than plasma levels achieved by clinically effective dosing, the anti-CTLA-4 antibody Ipilimumab blocks neither B7 trans-endocytosis by CTLA-4 nor CTLA-4 binding to immobilized or cell-associated B7. Consequently, Ipilimumab does not increase B7 on dendritic cells (DCs) from either CTLA4 gene humanized (Ctla4h/h) or human CD34+ stem cell-reconstituted NSG™ mice. In Ctla4h/m mice expressing both human and mouse CTLA4 genes, anti-CTLA-4 antibodies that bind to human but not mouse CTLA-4 efficiently induce Treg depletion and Fc receptor-dependent tumor rejection. The blocking antibody L3D10 is comparable to the non-blocking Ipilimumab in causing tumor rejection. Remarkably, L3D10 progenies that lose blocking activity during humanization remain fully competent in inducing Treg depletion and tumor rejection. Anti-B7 antibodies that effectively block CD4 T cell activation and de novo CD8 T cell priming in lymphoid organs do not negatively affect the immunotherapeutic effect of Ipilimumab. Thus, clinically effective anti-CTLA-4 mAb causes tumor rejection by mechanisms that are independent of checkpoint blockade but dependent on the host Fc receptor. Our data call for a reappraisal of the CTLA-4 checkpoint blockade hypothesis and provide new insights for the next generation of safe and effective anti-CTLA-4 mAbs. -

Non-Small Cell Lung Cancer
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer Version 3.2020 — February 11, 2020 NCCN.org NCCN Guidelines for Patients® Continue Version 3.2020, 02/11/20 © 2020 National Comprehensive Cancer Network® (NCCN®), All rights reserved. NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN. NCCN Guidelines Index NCCN Guidelines Version 3.2020 Table of Contents Non-Small Cell Lung Cancer Discussion *David S. Ettinger, MD/Chair † Michael Dobelbower, MD, PhD § Gregory A. Otterson, MD † The Sidney Kimmel Comprehensive O'Neal Comprehensive Cancer Center at UAB The Ohio State University Comprehensive Cancer Center at Johns Hopkins Cancer Center - James Cancer Hospital Scott Gettinger, MD † Þ and Solove Research Institute *Douglas E. Wood, MD/Vice Chair ¶ Yale Cancer Center/Smilow Cancer Hospital Fred Hutchinson Cancer Research Center/ Ramaswamy Govindan, MD † Sandip P. Patel, MD ‡ † Þ Seattle Cancer Care Alliance Siteman Cancer Center at Barnes- UC San Diego Moores Cancer Center Dara L. Aisner, MD, PhD ≠ Jewish Hospital and Washington Gregory J. Riely, MD, PhD † Þ University of Colorado Cancer Center University School of Medicine Memorial Sloan Kettering Cancer Center Wallace Akerley, MD † Matthew A. Gubens, MD, MS † Steven E. Schild, MD § Huntsman Cancer Institute UCSF Helen Diller Family Mayo Clinic Cancer Center at the University of Utah Comprehensive Cancer Center Theresa A. Shapiro, MD, PhD ¥ Þ Jessica R. Bauman, MD ‡ † Mark Hennon, MD ¶ The Sidney Kimmel Comprehensive Fox Chase Cancer Center Roswell Park Comprehensive Cancer Center Cancer Center at Johns Hopkins Ankit Bharat, MD ¶ Leora Horn, MD, MSc † Aditi P. -

CTLA-4 Blockade Reverses the Foxp3+ T-Regulatory-Cell Suppression of Anti-Tuberculosis T-Cell Effector Responses
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.11.089946; this version posted May 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. CTLA-4 blockade reverses the Foxp3+ T-regulatory-cell suppression of anti-tuberculosis T-cell effector responses Lingyun Shao1#, Yan Gao1#, Xiaoyi Shao 2#, Qingfang Ou3, Shu Zhang1,Qianqian Liu1, Bingyan Zhang1, Jing Wu1, Qiaoling Ruan1, Ling Shen5, Xinhua Weng1, Wenhong Zhang1,4,5,6 *, Zheng W. Chen7 1 Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai 200040, China 2 Department of Immunology, Medical College, Nantong University, Nantong 226001, China 3 Department of Pulmonary Diseases, Wuxi No.5 People’s Hospital, Wuxi 214005, China 4 Key Laboratory of Medical Molecular Virology (MOE/MOH) and Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China 5 State Key Laboratory of Genetic Engineering, School of Life Science, Fudan University, Shanghai 200438, China. 6 National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai 200040, China 7 Department of Microbiology & Immunology, Center for Primate Biomedical Research, University of Illinois College of Medicine, Chicago, IL 60612 #These authors contributed equally to this work. * Correspondencing author : Wenhong Zhang: 12 Wulumuqizhong Road, Shanghai 200040, China (e-mail: [email protected]). Running title: Anti-CTLA-4 reverses Treg function in TB 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.05.11.089946; this version posted May 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -
In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and in Vivo Toxicology in Non-Human Primates
Published OnlineFirst May 28, 2014; DOI: 10.1158/2326-6066.CIR-14-0040 Cancer Research Article Immunology Research In Vitro Characterization of the Anti-PD-1 Antibody Nivolumab, BMS-936558, and In Vivo Toxicology in Non-Human Primates Changyu Wang1, Kent B. Thudium1, Minhua Han1, Xi-Tao Wang1, Haichun Huang1, Diane Feingersh1, Candy Garcia1,YiWu1, Michelle Kuhne1, Mohan Srinivasan1, Sujata Singh1, Susan Wong1, Neysa Garner1, Heidi Leblanc1, R. Todd Bunch2, Diann Blanset3, Mark J. Selby1, and Alan J. Korman1 Abstract The programmed death-1 (PD-1) receptor serves as an immunologic checkpoint, limiting bystander tissue damage and preventing the development of autoimmunity during inflammatory responses. PD-1 is expressed by activated T cells and downmodulates T-cell effector functions upon binding to its ligands, PD-L1 and PD-L2, on antigen-presenting cells. In patients with cancer, the expression of PD-1 on tumor-infiltrating lymphocytes and its interaction with the ligands on tumor and immune cells in the tumor microenvironment undermine antitumor immunity and support its rationale for PD-1 blockade in cancer immunotherapy. This report details the development and characterization of nivolumab, a fully human IgG4 (S228P) anti-PD-1 receptor-blocking monoclonal antibody. Nivolumab binds to PD-1 with high affinity and specificity, and effectively inhibits the interaction between PD-1 and its ligands. In vitro assays demonstrated the ability of nivolumab to potently enhance T-cell responses and cytokine production in the mixed lymphocyte reaction and superantigen or cytomegalovirus stimulation assays. No in vitro antibody-dependent cell-mediated or complement-dependent cytotoxicity was observed with the use of nivolumab and activated T cells as targets. -

Controlled Local Delivery of CTLA-4 Blocking Antibody Induces CD8+ T Cell Dependent
Author Manuscript Published OnlineFirst on June 20, 2013; DOI: 10.1158/1078-0432.CCR-12-0781 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T cell dependent tumor eradication and decreases risk of toxic side-effects Marieke F. Fransen1, Tetje C. van der Sluis1, Ferry Ossendorp1, Ramon Arens1, Cornelis J. M. Melief1,2 1Department of Immunohematology and Blood Transfusion, Leiden University Medical Hospital, Leiden, the Netherlands. 2ISA Pharmaceuticals, Leiden, the Netherlands. Running title: Low dose CTLA-4 blocking antibody treatment Keywords: Anti tumor immunity, CD8+ T cells, Cytotoxic T-Lymphocyte Antigen-4, slow- release delivery Corresponding author: Cornelis JM Melief, Department of Immunohe- matology and Blood Transfusion, Leiden University Medical Center, 2300 RC, Leiden, The Netherlands. Phone: 31-715263800; Fax: 011-31- 715216751. E-mail: [email protected] Word count: 3297, 5 Figures. 1 Downloaded from clincancerres.aacrjournals.org on September 30, 2021. © 2013 American Association for Cancer Research. Author Manuscript Published OnlineFirst on June 20, 2013; DOI: 10.1158/1078-0432.CCR-12-0781 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Statement of translational relevance: Systemic delivery of CTLA-4 blocking antibodies induces anti-tumor immune responses in pre-clinical models and patients but dose-limiting toxicity hampers clinical success. We have used a novel delivery system based on the slow-release agent Montanide ISA-51 to distribute CTLA-4 blocking antibody in the lymphoid drainage area of the tumor, which stimulates local but not systemic T cells. -

The Role of T Cell Trafficking in CTLA-4 Blockade-Induced Gut
Zhang et al. BMC Biology (2020) 18:29 https://doi.org/10.1186/s12915-020-00765-9 RESEARCH ARTICLE Open Access The role of T cell trafficking in CTLA- 4 blockade-induced gut immunopathology Shashuang Zhang1,2, Wenhua Liang1,2, Lingjie Luo1,2, Shan Sun1,2 and Feng Wang1,2* Abstract Background: Immune checkpoint inhibitor (ICPI) can augment the anti-tumour response by blocking negative immunoregulators with monoclonal antibodies. The anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibody is the first ICPI which has shown remarkable benefits in the clinical treatment of cancers. However, the increased activity of the immune system also causes some side effects called immune-related adverse events (irAEs). Colitis is one of the most common irAEs related to anti-CTLA-4 immunotherapy. Results: We identified that CD4+ T cells were the primary responders in CTLA-4 blockade and that the expansion of gut-homing CD4+ T cells by anti-CTLA-4 therapy was independent of CD103. We used dextran sulfate sodium (DSS)-induced colitis mice as our model and tested the possibility of using a trafficking-blocking antibody to treat anti-CTLA-4 antibody-induced irAEs. We found that blocking T cell homing increased colitis severity in the context of CTLA-4 blockade and that gut-trafficking blockade had different effects on different Th subsets and could facilitate the proliferation of Th17 cells in the lamina propria (LP). Conclusions: Our data reveals the fundamental mechanism underlying trafficking-blocking antibody therapy for CTLA-4 blockade-induced colitis and provide a caution in regard to apply trafficking-blocking antibody treatment under CTLA-4 blockade condition.