Mathematical Description of Eukaryotic Chromosome Replication
Total Page:16
File Type:pdf, Size:1020Kb
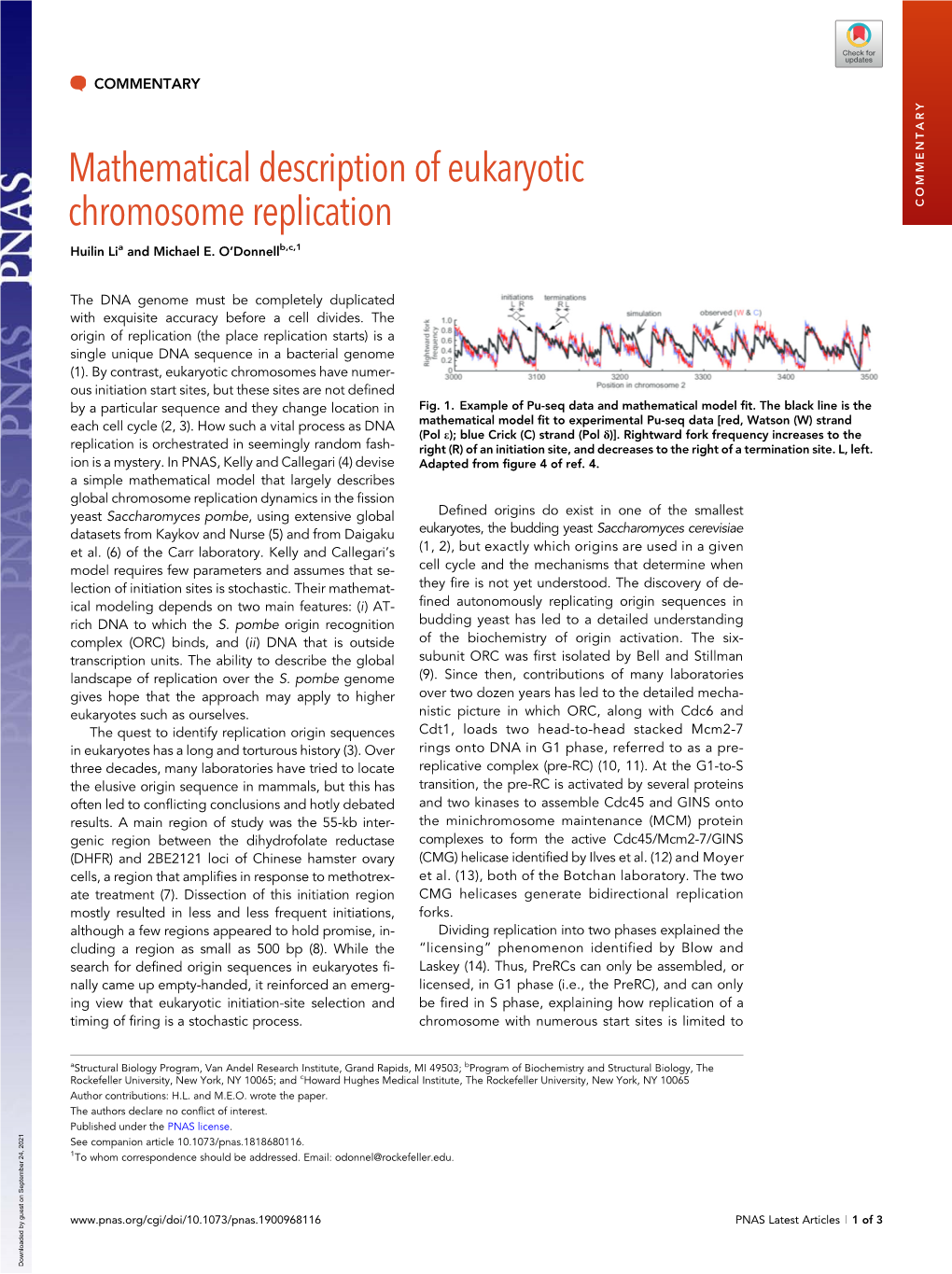
Load more
Recommended publications
-

Completion of DNA Replication in Escherichia Coli
Completion of DNA replication in Escherichia coli Brian M. Wendel1, Charmain T. Courcelle, and Justin Courcelle Department of Biology, Portland State University, Portland, OR 97201 Edited by Philip C. Hanawalt, Stanford University, Stanford, CA, and approved September 29, 2014 (received for review August 5, 2014) The mechanism by which cells recognize and complete replicated would generate a third copy of the chromosomal region where the regions at their precise doubling point must be remarkably efficient, event occurs. Thus, over-replication may be inherent and pro- occurring thousands of times per cell division along the chromo- miscuous during the duplication of genomes. If true, then to somes of humans. However, this process remains poorly understood. ensure that each sequence of the genome replicates once, and Here we show that, in Escherichia coli, the completion of replication only once, per generation, cells must encode an enzymatic system involves an enzymatic system that effectively counts pairs and limits that is essentially able to count in pairs and efficiently degrade cellular replication to its doubling point by allowing converging rep- odd or over-replicated regions until the two nascent end pairs of lication forks to transiently continue through the doubling point replication events can be joined. before the excess, over-replicated regions are incised, resected, and The model organism E. coli is particularly well-suited to dis- joined. Completion requires RecBCD and involves several proteins sect how this fundamental process occurs. In E. coli, the com- associated with repairing double-strand breaks including, ExoI, pletion of replication occurs at a defined region on the genome, SbcDC, and RecG. -

Mapping Major Replication Origins on the Rice Plastid DNA
27 Original Paper Plant Biotechnotogy, 19 (1), 27- 35 (2002) Mapping Major Replication Origins on the Rice Plastid DNA Ying WANG1, Kohya TAMURA2, Yasushi SAITOHl'2, Tadashi SAT03 Soh HIDAKA4 and Ken- ichi TSUTSUM11,2,* lUnited Graduate School ofAgricultural Sciences and -~Cryobiosystem Research Center, lwate University, Ueda, Morioka. Iwate 020- 8550, Japan 3Department of Ecology and Evolutionary Biology, Graduate School ofLlfe Science, Tohoku University, Katahira. Sendai, Miyagi 980- 8577, Japan dDepartment of Crop Breeding, National Agricultural Research Center for Tohoku Region, Shimokuriyagawa, Morioka, Iwate 020-0123, Japan. *Corresponding author E-mail address: kentsu@iwate- u.ac.jp Received 5september 2001; accepted 15 october 2001 Abstract To maintain and to differentiate into various plastid lineages, replication of the plastid DNA (ptDNA) and division of the plastid must take place. However, replication initiation of the ptDNA has been less understood. The present study describes identification of the initiation region (origin) of ptDNA replication in the rice cultured cells. RNA- primed newly replicated DNA strands pulse - Iabeled with fractionated. of these strands the bromodeoxyuridine were isolated and size - Locations nascent on ptDNA determined the two major origin regions around the 3' region of each 23S rDNA in the inverted gel electrophoresis of the replication intermediates repeats (IRA and IRB). Two - dimensional agarose suggested that replication from each origin proceeds bidirectionally. This contrasted to replication -

Γboris: Identification of Origins of Replication In
bioRxiv preprint doi: https://doi.org/10.1101/597070; this version posted April 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. γBOriS: Identification of Origins of Replication in Gammaproteobacteria using Motif-based Machine Learning Theodor Sperlea1, Lea Muth1, Roman Martin1, Christoph Weigel2, Torsten Waldminghaus3, and Dominik Heider1, 1Faculty of Mathematics and Computer Science, Philipps-Universität Marburg, D-35043 Marburg, Germany 2Institute of Biotechnology, Faculty III, Technische Universität Berlin (TUB), Straße des 17. Juni 135, D-10623 Berlin, Germany 3Chromosome Biology Group, LOEWE Center for Synthetic Microbiology (SYNMIKRO), Philipps-Universität Marburg, D-35043 Marburg, Germany The biology of bacterial cells is, in general, based on the infor- All currently available computational methods for the iden- mation encoded on circular chromosomes. Regulation of chro- tification of oriC sequences in bacterial chromosomes rely mosome replication is an essential process which mostly takes on nucleotide disparities on the leading and lagging strand place at the origin of replication (oriC). Identification of high of the DNA double helix (13–17). As replication usually ex- numbers of oriC is a prerequisite to enable systematic stud- tends from oriC bidirectionally, it is one of two chromoso- ies that could lead to insights of oriC functioning as well as mal sites where the leading and lagging strand switch places. novel drug targets for antibiotic development. Current meth- The most frequently used disparity, the GC skew, usually as- ods for identyfing oriC sequences rely on chromosome-wide nu- cleotide disparities and are therefore limited to fully sequenced sumes a V- or inverted V-shape with its minimum indicating genomes, leaving a superabundance of genomic fragments un- the presence of the origin of replication (18, 19). -

Initiating Chromosome Replication in E. Coli: It Makes Sense to Recycle
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press PERSPECTIVE Initiating chromosome replication in E. coli: it makes sense to recycle Alan C. Leonard1 and Julia E. Grimwade2 Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901, USA Initiating new rounds of Escherichia coli chromosome Escherichia coli initiator DnaA: building a bacterial replication requires DnaA-ATP to unwind the replication ORC and pre-RC origin, oriC, and load DNA helicase. In this issue of Questions about cycling initiator activity are particularly Genes & Development, Fujimitsu and colleagues (pp. relevant to DNA replication control in rapidly growing 1221–1233) demonstrate that two chromosomal sites, bacteria, since these cells contain multiple origins that termed DARS (DnaA-reactivating sequences), recycle must fire synchronously only once per cell division cycle inactive DnaA-ADP into DnaA-ATP. Fujimitsu and col- (Skarstad et al. 1986; Boye et al. 2000). Studies of the E. leagues propose these sites are necessary to attain the coli AAA+ initiator, DnaA protein, provide insights into DnaA-ATP threshold during normal growth and are im- portant regulators of initiation timing in bacteria. the various cellular mechanisms that ensure that the initiator is active and available at the time of initiation and is then prevented from triggering another round of replication until the next cell cycle. DnaA has a high All cells must coordinate the timing of chromosome affinity for adenine nucleotides ADP and ATP, but is duplication with cell division during the cell cycle, to active only when bound to ATP (Sekimizu et al. -

Occlusion of the HIV Poly(A) Site
Downloaded from genesdev.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press Occlusion of the HIV poly(A) site Caroline Weichs an der Glon, Joan Monks, and Nick J. Proudfoot Sir William Dunn School of Pathology, University of Oxford, Oxford OXl 3RE UK To investigate the selective use of poly{A) sites in the 3' long terminal repeat (LTR) but not the 5' LTR of retroviruses, we have studied the poly (A) site of the human immunodeficiency virus (HIV-1). Using hybrid HIV/a-globin gene constructs, we demonstrate that the HIV poly(A) site is inactive or occluded when adjacent to an active promoter, either the homologous HIV promoter or the a-globin gene promoter. Furthermore, this occlusion of the HIV poly(A) site occurs over a considerable distance of up to at least 500 bp. In contrast, two nonretroviral poly(A) sites [a-globin and a synthetic poly{A) site] are active when close to a promoter. We also show that a short fragment of -60 nucleotides containing the HIV poly(A) site is fully active when placed at the 3' end of the human a-globin gene or within the rabbit p-globin gene. This result rules out the requirement of more distant upstream elements for the activity of the HIV poly(A) site, as has been suggested for other viral poly(A) sites. Finally, we show that the GT-rich downstream region of the HIV poly(A) site confers poly{A) site occlusion properties on a synthetic poly(A) site. This result focuses attention on this more variable part of a poly(A) site in retrovirvses as a possible general signal for poly(A) site occlusion. -

Pcor: a New Design of Plasmid Vectors for Nonviral Gene Therapy
Gene Therapy (1999) 6, 1482–1488 1999 Stockton Press All rights reserved 0969-7128/99 $12.00 http://www.stockton-press.co.uk/gt BRIEF COMMUNICATION pCOR: a new design of plasmid vectors for nonviral gene therapy F Soubrier, B Cameron, B Manse, S Somarriba, C Dubertret, G Jaslin, G Jung, C Le Caer, D Dang, JM Mouvault, D Scherman, JF Mayaux and J Crouzet Rhoˆne-Poulenc Rorer, Centre de Recherche de Vitry Alfortville, 13 Quai J Guesde, 94403 Vitry-sur-Seine, France A totally redesigned host/vector system with improved initiator protein, protein, encoded by the pir gene limiting properties in terms of safety has been developed. The its host range to bacterial strains that produce this trans- pCOR plasmids are narrow-host range plasmid vectors for acting protein; (2) the plasmid’s selectable marker is not an nonviral gene therapy. These plasmids contain a con- antibiotic resistance gene but a gene encoding a bacterial ditional origin of replication and must be propagated in a suppressor tRNA. Optimized E. coli hosts supporting specifically engineered E. coli host strain, greatly reducing pCOR replication and selection were constructed. High the potential for propagation in the environment or in yields of supercoiled pCOR monomers were obtained (100 treated patients. The pCOR backbone has several features mg/l) through fed-batch fermentation. pCOR vectors carry- that increase safety in terms of dissemination and selec- ing the luciferase reporter gene gave high levels of lucifer- tion: (1) the origin of replication requires a plasmid-specific ase activity when injected into murine skeletal muscle. Keywords: gene therapy; plasmid DNA; conditional replication; selection marker; multimer resolution Two different types of DNA vehicles, based criteria.4 These high copy number plasmids carry a mini- on recombinant viruses and bacterial DNA plasmids, are mal amount of bacterial sequences, a conditional origin used in gene therapy. -

Extrachromosomal Element Capture and the Evolution of Multiple Replication Origins in Archaeal Chromosomes
Extrachromosomal element capture and the evolution of multiple replication origins in archaeal chromosomes Nicholas P. Robinson† and Stephen D. Bell† Medical Research Council Cancer Cell Unit, Hutchison Medical Research Council Research Center, Hills Road, Cambridge CB2 0XZ, United Kingdom Edited by Carl R. Woese, University of Illinois at Urbana–Champaign, Urbana, IL, and approved February 15, 2007 (received for review January 9, 2007) In all three domains of life, DNA replication begins at specialized Orc2–6 act to recruit MCM to origins of replication in a reaction loci termed replication origins. In bacteria, replication initiates from that absolutely requires an additional factor, Cdt1 (6). Although a single, clearly defined site. In contrast, eukaryotic organisms archaea possess orthologs of Orc1, Cdc6, and MCM, no archaeal exploit a multitude of replication origins, dividing their genomes homolog of Cdt1 has yet been identified. into an array of short contiguous units. Recently, the multiple In the current work, we reveal that Aeropyrum pernix has at replication origin paradigm has also been demonstrated within the least two replication origins, indicating that the multiple repli- archaeal domain of life, with the discovery that the hyperthermo- cation origin paradigm is not restricted to the Sulfolobus genus. philic archaeon Sulfolobus has three replication origins. However, Comparison of the A. pernix and Sulfolobus origins reveals a clear the evolutionary mechanism driving the progression from single to relationship between these loci. Further, analyses of the gene multiple origin usage remains unclear. Here, we demonstrate that order and identity in the environment of the origins provides Aeropyrum pernix, a distant relative of Sulfolobus, has two origins. -

Diversity of DNA Replication in the Archaea
G C A T T A C G G C A T genes Review Diversity of DNA Replication in the Archaea Darya Ausiannikava * and Thorsten Allers School of Life Sciences, University of Nottingham, Nottingham NG7 2UH, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-115-823-0304 Academic Editor: Eishi Noguchi Received: 29 November 2016; Accepted: 20 January 2017; Published: 31 January 2017 Abstract: DNA replication is arguably the most fundamental biological process. On account of their shared evolutionary ancestry, the replication machinery found in archaea is similar to that found in eukaryotes. DNA replication is initiated at origins and is highly conserved in eukaryotes, but our limited understanding of archaea has uncovered a wide diversity of replication initiation mechanisms. Archaeal origins are sequence-based, as in bacteria, but are bound by initiator proteins that share homology with the eukaryotic origin recognition complex subunit Orc1 and helicase loader Cdc6). Unlike bacteria, archaea may have multiple origins per chromosome and multiple Orc1/Cdc6 initiator proteins. There is no consensus on how these archaeal origins are recognised—some are bound by a single Orc1/Cdc6 protein while others require a multi- Orc1/Cdc6 complex. Many archaeal genomes consist of multiple parts—the main chromosome plus several megaplasmids—and in polyploid species these parts are present in multiple copies. This poses a challenge to the regulation of DNA replication. However, one archaeal species (Haloferax volcanii) can survive without replication origins; instead, it uses homologous recombination as an alternative mechanism of initiation. This diversity in DNA replication initiation is all the more remarkable for having been discovered in only three groups of archaea where in vivo studies are possible. -

Replication of Mitochondrial DNA Occurs by Strand Displacement with Alternative Light-Strand Origins, Not Via a Strand-Coupled Mechanism
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism Timothy A. Brown,1,2,6 Ciro Cecconi,3 Ariana N. Tkachuk,1,2 Carlos Bustamante,4 and David A. Clayton1,5 1Howard Hughes Medical Institute, Chevy Chase, Maryland 20815, USA; 2National Institutes of Health, Guest, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland 20892, USA; 3Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA; 4Howard Hughes Medical Institute, Department of Molecular and Cell Biology and Department of Physics, University of California, Berkeley, Berkeley, California 94720, USA The established strand-displacement model for mammalian mitochondrial DNA (mtDNA) replication has recently been questioned in light of new data using two-dimensional (2D) agarose gel electrophoresis. It has been proposed that a synchronous, strand-coupled mode of replication occurs in tissues, thereby casting doubt on the general validity of the “orthodox,” or strand-displacement model. We have examined mtDNA replicative intermediates from mouse liver using atomic force microscopy and 2D agarose gel electrophoresis in order to resolve this issue. The data provide evidence for only the orthodox, strand-displacement mode of replication and reveal the presence of additional, alternative origins of lagging light-strand mtDNA synthesis. The conditions used for 2D agarose gel analysis are favorable for branch migration of asymmetrically replicating nascent strands. These data reconcile the original displacement mode of replication with the data obtained from 2D gel analyses. [Keywords: Replication; mtDNA; branch migration; atomic force microscopy; 2D agarose gels; strand displacement] Supplemental material is available at http://www.genesdev.org. -

Origin and Direction of Replication of the Chromosome of E. Coli B/R Millicent Masters
Proceedings of the National Academy of Sciences Vol. 65, No. 3 pp. 601-608, March 1970 Origin and Direction of Replication of the Chromosome of E. coli B/r Millicent Masters MEDICAL RESEARCH COUNCIL, MICROBIAL GENETICS RESEARCH UNIT, DEPARTMENT OF MOLECULAR BIOLOGY, EDINBURGH UNIVERSITY, EDINBURGH, SCOTLAND Communicated by Arthur B. Pardee, July 3, 1969 Abstract. The origin and direction of replication of the E. coli B/r chromo- some has been determined by comparing gene frequencies in Pl-transducing lysates prepared on cultures growing at different rates. The gene frequencies found are consistent with the idea that replication of the chromosome is di- chotomous in rapidly growing B/r. The origin was found to be between 40 and 55 min on the E. coli genetic map with replication proceeding in a clockwise direction. Markers near the origin behaved anomalously. The chromosome of E. coli is a single circular molecule of DNA which replicates sequentially along its length.'-3 Since the demonstration by Yoshikawa and Sueoka4-5 that the B. subtilis chromosome replicates sequentially from a genet- ically fixed origin, many attempts have been made to determine whether this is also true for the circular chromosome of E. coli. Although some early work6-7 indicated that the presence and position of such an origin might be dependent on the integration of the F sex factor, later work has supported the idea that the E. coli chromosome is always replicated sequentially in a particular direction from a fixed origin whose position may be strain dependent, but probably does not de- pend on the integration of the F factor.8-'5 The majority of strains which have been studied are estimated to have an origin lying between 40 and 70 min on the genetic transfer map of E. -

Manual: Pfb and Pfb-Neo Retroviral Vectors
pFB and pFB-Neo Retroviral Vectors INSTRUCTION MANUAL Catalog #217563 (pFB Retroviral Vector) and #217561 (pFB-Neo Retroviral Vector) Revision A For In Vitro Use Only 217561-12 LIMITED PRODUCT WARRANTY This warranty limits our liability to replacement of this product. No other warranties of any kind, express or implied, including without limitation, implied warranties of merchantability or fitness for a particular purpose, are provided by Agilent. Agilent shall have no liability for any direct, indirect, consequential, or incidental damages arising out of the use, the results of use, or the inability to use this product. ORDERING INFORMATION AND TECHNICAL SERVICES United States and Canada Agilent Technologies Stratagene Products Division 11011 North Torrey Pines Road La Jolla, CA 92037 Telephone (858) 373-6300 Order Toll Free (800) 424-5444 Technical Services (800) 894-1304 Internet [email protected] World Wide Web www.stratagene.com Europe Location Telephone Fax Technical Services Austria 0800 292 499 0800 292 496 0800 292 498 Belgium 00800 7000 7000 00800 7001 7001 00800 7400 7400 0800 15775 0800 15740 0800 15720 France 00800 7000 7000 00800 7001 7001 00800 7400 7400 0800 919 288 0800 919 287 0800 919 289 Germany 00800 7000 7000 00800 7001 7001 00800 7400 7400 0800 182 8232 0800 182 8231 0800 182 8234 Netherlands 00800 7000 7000 00800 7001 7001 00800 7400 7400 0800 023 0446 +31 (0)20 312 5700 0800 023 0448 Switzerland 00800 7000 7000 00800 7001 7001 00800 7400 7400 0800 563 080 0800 563 082 0800 563 081 United Kingdom 00800 7000 7000 00800 7001 7001 00800 7400 7400 0800 917 3282 0800 917 3283 0800 917 3281 All Other Countries Please contact your local distributor. -

Origin of Replication - I
Origin of Replication - I Signals and Systems in Biology Kushal Shah @ EE, IIT Delhi DNA Replication Bacterial DNA Ori : Some facts I Replication may proceed uni-directionally or bi-directionally I Usually AT-rich region I Bacteria : circular DNA and single origin of replication I Archaea : Circular DNA and multiple origins I Eukaryotes : Linear DNA and multiple origins I Firing time of each Ori may be different I Modeling of this ’firing’ phenomenon is a challenging task Ori : Some facts I Replication may proceed uni-directionally or bi-directionally I Usually AT-rich region I Bacteria : circular DNA and single origin of replication I Archaea : Circular DNA and multiple origins I Eukaryotes : Linear DNA and multiple origins I Firing time of each Ori may be different I Modeling of this ’firing’ phenomenon is a challenging task Ori : Some facts I Replication may proceed uni-directionally or bi-directionally I Usually AT-rich region I Bacteria : circular DNA and single origin of replication I Archaea : Circular DNA and multiple origins I Eukaryotes : Linear DNA and multiple origins I Firing time of each Ori may be different I Modeling of this ’firing’ phenomenon is a challenging task Ori : Some facts I Replication may proceed uni-directionally or bi-directionally I Usually AT-rich region I Bacteria : circular DNA and single origin of replication I Archaea : Circular DNA and multiple origins I Eukaryotes : Linear DNA and multiple origins I Firing time of each Ori may be different I Modeling of this ’firing’ phenomenon is a challenging task Ori