Identifying Seaweed Consumption by Sheep Using Isotope Analysis Of
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
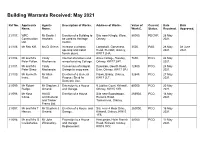
Building Warrants Received: May 2021
Building Warrants Received: May 2021 Ref No. Applicants Agents Description of Works. Address of Works. Value of Current Date Date Name. Name. Work £. Status. Received. Approved. 21/107. WRC Mr Bashir I Erection of a Building to Site near Kringlo, Wyre, 85000. RECNP. 28 May Construction Hasham. be used as Heritage Orkney. 2021. Ltd. Centre. 21/106. Mr Rob Kiff. Ms Di Grieve. Increase a window Langskaill, Gorseness 3500. PAS. 28 May 08 June opening and install Road, Rendall, Orkney, 2021. 2021. french doors. KW17 2HA. 21/104. Mr and Mrs Cindy Internal alterations and Alma Cottage, Sanday, 7600. PCO. 24 May Peter Fallon. Mackenzie. re-roof existing Cottage. Orkney, KW17 2AY. 2021. 21/101. Mr and Mrs Cindy Conversion of integral Dykeside, Georth Road, 12500. PCO. 24 May Peter Shaw. Mackenzie. Garage to snug area. Evie, Orkney, KW17 2PJ. 2021. 21/100. Mr Kenneth Mr Allan Erection of a General Flaws, Birsay, Orkney, 32848. PCO. 27 May Irvine. Reid. Purpose Shed for KW17 2LT. 2021. Domestic Use. 21/099. Mr Robert Mr Stephen J Extension to a House 9 Jubilee Court, Kirkwall, 80000. PCO. 20 May Budge. Omand. and Garage. Orkney, KW15 1XR. 2021. 21/098. Mr Ross HAUS Erection of a House. Site near Boondatoon, 266902. PCO. 18 May Corse. Architectural Rerwick Road, 2021. and Timber Tankerness, Orkney. Frame Ltd. 21/097. Mr and Mrs T Mr Stephen J Erection of a House and Site near 4 Moar Drive, 260000. PCO. 18 May Harcus. Omand. Garage. Kirkwall, Orkney, KW15 2021. 1FS. 21/096. Mr and Mrs G Mr John Extension to a House Hescombe, Holm Branch 30000. -

Ferry Timetables
1768 Appendix 1. www.orkneyferries.co.uk GRAEMSAY AND HOY (MOANESS) EFFECTIVE FROM 24 SEPTEMBER 2018 UNTIL 4 MAY 2019 Our service from Stromness to Hoy/Graemsay is a PASSENGER ONLY service. Vehicles can be carried by prior arrangement to Graemsay on the advertised cargo sailings. Monday Tuesday Wednesday Thursday Friday Saturday Sunday Stromness dep 0745 0745 0745 0745 0745 0930 0930 Hoy (Moaness) dep 0810 0810 0810 0810 0810 1000 1000 Graemsay dep 0825 0825 0825 0825 0825 1015 1015 Stromness dep 1000 1000 1000 1000 1000 Hoy (Moaness) dep 1030 1030 1030 1030 1030 Graemsay dep 1045 1045 1045 1045 1045 Stromness dep 1200A 1200A 1200A Graemsay dep 1230A 1230A 1230A Hoy (Moaness) dep 1240A 1240A 1240A Stromness dep 1600 1600 1600 1600 1600 1600 1600 Graemsay dep 1615 1615 1615 1615 1615 1615 1615 Hoy (Moaness) dep 1630 1630 1630 1630 1630 1630 1630 Stromness dep 1745 1745 1745 1745 1745 Graemsay dep 1800 1800 1800 1800 1800 Hoy (Moaness) dep 1815 1815 1815 1815 1815 Stromness dep 2130 Graemsay dep 2145 Hoy (Moaness) dep 2200 A Cargo Sailings will have limitations on passenger numbers therefore booking is advisable. These sailings may be delayed due to cargo operations. Notes: 1. All enquires must be made through the Kirkwall Office. Telephone: 01856 872044. 2. Passengers are requested to be available for boarding 5 minutes before departure. 3. Monday cargo to be booked by 1600hrs on previous Friday otherwise all cargo must be booked before 1600hrs the day before sailing. Cargo must be delivered to Stromness Pier no later than 1100hrs on the day of sailing. -

National Retailers.Xlsx
THE NATIONAL / SUNDAY NATIONAL RETAILERS Store Name Address Line 1 Address Line 2 Address Line 3 Post Code M&S ABERDEEN E51 2-28 ST. NICHOLAS STREET ABERDEEN AB10 1BU WHS ST NICHOLAS E48 UNIT E5, ST. NICHOLAS CENTRE ABERDEEN AB10 1HW SAINSBURYS E55 UNIT 1 ST NICHOLAS CEN SHOPPING CENTRE ABERDEEN AB10 1HW RSMCCOLL130UNIONE53 130 UNION STREET ABERDEEN, GRAMPIAN AB10 1JJ COOP 204UNION E54 204 UNION STREET X ABERDEEN AB10 1QS SAINSBURY CONV E54 SOFA WORKSHOP 206 UNION STREET ABERDEEN AB10 1QS SAINSBURY ALF PL E54 492-494 UNION STREET ABERDEEN AB10 1TJ TESCO DYCE EXP E44 35 VICTORIA STREET ABERDEEN AB10 1UU TESCO HOLBURN ST E54 207 HOLBURN STREET ABERDEEN AB10 6BL THISTLE NEWS E54 32 HOLBURN STREET ABERDEEN AB10 6BT J&C LYNCH E54 66 BROOMHILL ROAD ABERDEEN AB10 6HT COOP GT WEST RD E46 485 GREAT WESTERN ROAD X ABERDEEN AB10 6NN TESCO GT WEST RD E46 571 GREAT WESTERN ROAD ABERDEEN AB10 6PA CJ LANG ST SWITIN E53 43 ST. SWITHIN STREET ABERDEEN AB10 6XL GARTHDEE STORE 19-25 RAMSAY CRESCENT GARTHDEE ABERDEEN AB10 7BL SAINSBURY PFS E55 GARTHDEE ROAD BRIDGE OF DEE ABERDEEN AB10 7QA ASDA BRIDGE OF DEE E55 GARTHDEE ROAD BRIDGE OF DEE ABERDEEN AB10 7QA SAINSBURY G/DEE E55 GARTHDEE ROAD BRIDGE OF DEE ABERDEEN AB10 7QA COSTCUTTER 37 UNION STREET ABERDEEN AB11 5BN RS MCCOLL 17UNION E53 17 UNION STREET ABERDEEN AB11 5BU ASDA ABERDEEN BEACH E55 UNIT 11 BEACH BOULEVARD RETAIL PARK LINKS ROAD, ABERDEEN AB11 5EJ M & S UNION SQUARE E51 UNION SQUARE 2&3 SOUTH TERRACE ABERDEEN AB11 5PF SUNNYS E55 36-40 MARKET STREET ABERDEEN AB11 5PL TESCO UNION ST E54 499-501 -

The Kirk in the Garden of Evie
THE KIRK IN THE GARDEN OF EVIE A Thumbnail Sketch of the History of the Church in Evie Trevor G Hunt Minister of the linked Churches of Evie, Firth and Rendall, Orkney First Published by Evie Kirk Session Evie, Orkney. 1987 Republished 1996 ComPrint, Orkney 908056 Forward to the 1987 Publication This brief history was compiled for the centenary of the present Evie Church building and I am indebted to all who have helped me in this work. I am especially indebted to the Kirk’s present Session Clerk, William Wood of Aikerness, who furnished useful local information, searched through old Session Minutes, and compiled the list of ministers for Appendix 3. Alastair Marwick of Whitemire, Clerk to the Board, supplied a good deal of literature, obtained a copy of the Title Deeds, gained access to the “Kirk aboon the Hill”, and conducted a tour (even across fields in his car) to various sites. He also contributed valuable local information and I am grateful for all his support. Thanks are also due to Margaret Halcro of Lower Crowrar, Rendall, for information about her name sake, and to the Moars of Crook, Rendall, for other Halcro family details. And to Sheila Lyon (Hestwall, Sandwick), who contributed information about Margaret Halcro (of the seventeenth century!). TREVOR G HUNT Finstown Manse March 1987 Foreword to the 1996 Publication Nearly ten years on seemed a good time to make this history available again, and to use the advances in computer technology to improve its appearance and to make one or two minor corrections.. I was also anxious to include the text of the history as a page on the Evie, Firth and Rendall Churches’ Internet site for reference and, since revision was necessary to do this, it was an opportunity to republish in printed form. -

The Significance of the Ancient Standing Stones, Villages, Tombs on Orkney Island
The Proceedings of the International Conference on Creationism Volume 5 Print Reference: Pages 561-572 Article 43 2003 The Significance of the Ancient Standing Stones, Villages, Tombs on Orkney Island Lawson L. Schroeder Philip L. Schroeder Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Schroeder, Lawson L. and Schroeder, Philip L. (2003) "The Significance of the Ancient Standing Stones, Villages, Tombs on Orkney Island," The Proceedings of the International Conference on Creationism: Vol. 5 , Article 43. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol5/iss1/43 THE SIGNIFICANCE OF THE ANCIENT STANDING STONES, VILLAGES AND TOMBS FOUND ON THE ORKNEY ISLANDS LAWSON L. SCHROEDER, D.D.S. PHILIP L. SCHROEDER 5889 MILLSTONE RUN BRYAN COLLEGE STONE MOUNTAIN, GA 30087 P. O. BOX 7484 DAYTON, TN 37321-7000 KEYWORDS: Orkney Islands, ancient stone structures, Skara Brae, Maes Howe, broch, Ring of Brodgar, Standing Stones of Stenness, dispersion, Babel, famine, Ice Age ABSTRACT The Orkney Islands make up an archipelago north of Scotland. -

Ring of Brodgar
Property in Care (PIC) ID: PIC313 Designations: Scheduled Monument (SM90042) Taken into State care: 1906 (Ownership) Last reviewed: 2018 STATEMENT OF SIGNIFICANCE RING OF BRODGAR We continually revise our Statements of Significance, so they may vary in length, format and level of detail. While every effort is made to keep them up to date, they should not be considered a definitive or final assessment of our properties. Historic Environment Scotland – Scottish Charity No. SC045925 Principal Office: Longmore House, Salisbury Place, Edinburgh EH9 1SH © Historic Environment Scotland 2018 You may re-use this information (excluding logos and images) free of charge in any format or medium, under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit http://nationalarchives.gov.uk/doc/open- government-licence/version/3/ or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. Any enquiries regarding this document should be sent to us at: Historic Environment Scotland Longmore House Salisbury Place Edinburgh EH9 1SH +44 (0) 131 668 8600 www.historicenvironment.scot You can download this publication from our website at www.historicenvironment.scot Historic Environment Scotland – Scottish Charity No. SC045925 Principal Office: Longmore House, Salisbury Place, Edinburgh EH9 1SH RING OF BRODGAR, STONE CIRCLE AND HENGE BRIEF DESCRIPTION The monument comprises a massive ceremonial enclosure, or ‘henge’; its rock-cut ditch (c.123m diameter) encircling a platform with an impressive stone circle set around its circumference. -

History of Medicine
HISTORY OF MEDICINE The air-ambulance: Orkney's experience R. A. COLLACOTT, MA, DM, PH.D, MRCGP RCGP History of General Practice Research Fellow; formerly General Practitioner, Isle of Westray, Orkney Islands SUMMARY. The paramount problem for the de- isolated medical service. Patients could be transferred livery of the medical services in the Orkneys has between islands and from the islands to mainland been that of effective transport. The develop- Scotland. It became easier for general practitioners to ment of an efficient air-ambulance service has obtain the assistance of colleagues in other islands, had a major impact on medical care. The service which led to more effective specialist services in the started in 1934, but was abolished at the outset of main island townships of Kirkwall in the Orkney Isles, the Second World War and did not recommence Stornoway in the Hebrides and Lerwick in the Shetland until 1967. This paper examines the evolution of Isles. The air-ambulance made attending regional cen- the air-ambulance service in the Orkney Islands, tres such as Aberdeen easier and more comfortable for and describes alternative proposals for the use of patients than the conventional, slower journey by boat: aircraft in this region. for example, the St Ola steamer took four to five hours to sail between Kirkwall and Wick via Thurso whereas the plane took only 35 minutes; furthermore, patients Introduction often became more ill as a result of the sea journey alone, the Pentland Firth being notorious for its stormy UNLIKE the other groups of Scottish islands, the I Orkney archipelago a of seas. -

In Vil<Ing Age Orkney
'Central places' in Vil<ing Age Orkney Frans-Arne Stylegar The present paper is an attenlpt to stinlulate discussion based on an analysis of the distribution patterns of S0111e place-names in Orkney. I It is argued, based on H. Mar\vick's interpretations, that SOlne of the Norse place-natnes in these islands seeln to belong to types that in Scandinavia are considered indicative of nodal or central places of the late Iron Age. The question is posed whether we in Viking Age Orkney can expect a social organisation and a settletnent structure similar to the one in the Scandinavian countries, and - if so - \vhat constitutes such a pattern? The Northern Isles lnay fulfil an itnportant role for students of Scandinavian central places, since one fronl the landnilJn situation in Orkney could, potentially, reach a fuller understanding of both chronological and social aspects of the different kinds of nodal places in the Scandinavian 'holne-lands'. Other parts of Britain, such as the Scottish Western Isles, could in principle serve the salne function, but in the latter case early Norse settletnent sites with only one exception still await discovery (Annit ]996). The study o.f·central places - so/ne Scandinavian examples Strictly speaking, the central place is an archaeological concept, denoting Iron Age settletnents with a rich and varied find material. Thus it covers sites that fulfilled various functions (Fabech 1999). The concept was reintroduced into Scandinavian archaeology after a symposiulll in Denlllark in 1989, first and foretnost to cOlne to tenns with a new type of Inetal-rich settlelnents that tnetal detector surveying had brought to light in Dennlark and Sweden (ibid.). -

Buxa Chalets Copy
B U X A F A R M CHALETS Self-Catering accommodations How to get here From Stromness: When departing the ferry in Stromness turn right upon departure from the ferry parking lot onto the A965 taking you towards Kirkwall. Take the 3rd left off the traffic circle and take a right at the end of the road. Turn right at A964 signposted to Orphir. Continue for approximately 5 miles and Buxa Farm Chalets is signposted on the right. Go to the end of the road and take a left at next signpost. Approximately 15 minutes. If What is there to see and do? using GPS use postcode: KW17 2RD From Kirkwall: When departing the ferry in Kirkwall turn left upon departure from the ferry parking lot and head through industrial park (taking you towards Kirkwall) to The most common complaint we get from visitors is junction, take left and then 2nd left off traffic circle at Mills that there just isn’t enough time to see everything in Filling Station and follow road to another traffic circle and Orkney! We suggest before you visit that you log on to take 2nd left. Take a right at the A964 Orphir road and the local tourist information site: stay on for about 20 minutes passing through small www: visitorkney.com that is just chock full of village of Orphir and uphill past Houton Ferry Terminal. information on what to see and do in Orkney. Buxa Farm Chalets is signposted on the left. Go to the Alternatively you can phone the VisitOrkney tourist end of the road and take a left at next signpost. -

Inter-Island Ferry Services – Proposed Summer 2020 Timetable
Item: 10 Development and Infrastructure Committee: 12 November 2019. Inter-Island Ferry Services – Proposed Summer 2020 Timetable. Report by Executive Director of Development and Infrastructure. 1. Purpose of Report To consider the proposed inter-island ferry services timetables for summer 2020. 2. Recommendations The Committee is invited to note: 2.1. That the inter-island ferry services timetables for summer 2020 are scheduled to operate from 3 May to 28 September 2020. 2.2. That draft timetables in respect of ferry services to be operated by Orkney Ferries Limited during summer 2020 were presented to the Ferry Services Consultative Forum for consideration on 21 August 2019, with main comments and representations from transport representatives outlined in section 4 of this report. 2.3. That, on 19 September 2019, the proposed timetables, together with feedback from the Ferry Services Consultative Forum, were considered by the Board of Orkney Ferries Limited and recommended to the Council for implementation. 2.4. That the proposed timetables, attached as Appendix 1 to this report, remain broadly consistent with those operated during summer 2019, including the Tuesday and Thursday return link from Eday to Sanday for education purposes, which was trialled during 2018 to 2019. 2.5. That, in advance of the Council’s budget setting process for 2020 to 2021 being concluded, any decision on the proposed Orkney Ferries summer timetable for 2020 will be subject to an adequate service revenue budget being established for financial year 2020 to 2021. Page 1. It is recommended: 2.6. That, subject to an adequate service revenue budget being established for financial year 2020 to 2021, the timetables in respect of ferry services to be operated by Orkney Ferries Limited during summer 2020, attached as Appendix 1 to this report, be approved. -

The Orkney Sheep Foundation
Vol 15 No 3 Paddocks and Perches Page 4 The Orkney Sheep Foundation By Kate Traill-Price The Orkney Sheep Foundation was founded at While other flocks in similar circumstances the start of 2015 with a clear objective: that of suffered at the chanGes and harsh conditions, “conserving an island heritage.” the wily sheep of North Ronaldsay not only adapted to their new habitat, but thrived The island in question is North Ronaldsay - a upon it. With the wild North Sea throwinG an tiny piece of land dotted at the very top of the abundance of seaweed onto archipelago known as the the shore, the sheep’s diet Orkney Islands, in became an oceanic feast of northernmost Scotland in nutrient-rich kelp. Unlike the United KinGdom. most other animals, the On this small, unassuminG North Ronaldsay sheep are island which counts less actually at their optimum than 50 residents, lives a weight in the deepest breed of sheep unlike any depths of winter, when the other in the world, and this sea’s fierce rip tides produce is the heritage that the OSF an even Greater bounty. is seekinG to conserve: the ancient breed of Each island crofter was entitled to keep a North Ronaldsay seaweed-eatinG sheep. select number of sheep on the shore, and in The flock’s bloodline Goes back thousands of exchanGe would help to rebuild and repair any years, with recent studies by the French section of sheepdyke that ran alonGside their Natural History Museum showinG that Orkney farminG land. sheep were supplementinG their diet with So it worked for hundreds of years but with seaweed as far back as 4000 BC. -

ACE Appendix
CBP and Trade Automated Interface Requirements Appendix: PGA August 13, 2021 Pub # 0875-0419 Contents Table of Changes .................................................................................................................................................... 4 PG01 – Agency Program Codes ........................................................................................................................... 18 PG01 – Government Agency Processing Codes ................................................................................................... 22 PG01 – Electronic Image Submitted Codes .......................................................................................................... 26 PG01 – Globally Unique Product Identification Code Qualifiers ........................................................................ 26 PG01 – Correction Indicators* ............................................................................................................................. 26 PG02 – Product Code Qualifiers ........................................................................................................................... 28 PG04 – Units of Measure ...................................................................................................................................... 30 PG05 – Scientific Species Code ........................................................................................................................... 31 PG05 – FWS Wildlife Description Codes ...........................................................................................................