Sogc 4 Bcphp Fetal Health Surveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pre-Term Pre-Labour Rupture of Membranes and the Role of Amniocentesis
Fetal and Maternal Medicine Review 2010; 21:2 75–88 C Cambridge University Press 2010 doi:10.1017/S096553951000001X First published online 15 March 2010 PRE-TERM PRE-LABOUR RUPTURE OF MEMBRANES AND THE ROLE OF AMNIOCENTESIS 1,2 ANNA P KENYON, 1,2 KHALIL N ABI-NADER AND 2 PRANAV P PANDYA 1Elizabeth Garrett Anderson Institute for Women’s Health, University College London, 86-96 Chenies Mews, London WCIE 6NX. 2Fetal Medicine Unit, University College London Hospitals NHS Foundation Trust, 235 Euston Rd, London NWI 2BU. INTRODUCTION Pre-labour premature rupture of membranes (PPROM) is defined as rupture of membranes more than 1 hour prior to the onset of labour at <37 weeks gestation. PPROM occurs in approximately 3% of pregnancies and is responsible for a third of all preterm births.1 Once membranes are ruptured prolonging the pregnancy has no maternal physical advantage but fetal morbidity and mortality are improved daily at early gestations: 19% of those infants born <25 weeks develop cerebral palsy (CP) and 28% have severe motor disability.2 Those infants born extremely pre term (<28 weeks) cost the public sector £75835 (95% CI £27906–145508) per live birth3 not to mention the emotional cost to the family. To prolong gestation is therefore the suggested goal: however how and why might we delay birth in those at risk? PPROM is one scenario associated with preterm birth and here we discuss the causative mechanisms, sequelae, latency, strategies to prolong gestation (antibiotics) and consider the role of amniocentesis. We will also discuss novel therapies. PATHOPHYSIOLOGY OF MEMBRANE RUPTURE The membranes, which act to protect and isolate the fetus, are composed of two layers. -

Characteristics of Heart Rate Tracings in Preterm Fetus
medicina Review Characteristics of Heart Rate Tracings in Preterm Fetus Maria F. Hurtado-Sánchez 1, David Pérez-Melero 2, Andrea Pinto-Ibáñez 3 , Ernesto González-Mesa 4 , Juan Mozas-Moreno 1,5,6,7,* and Alberto Puertas-Prieto 1,7 1 Obstetrics and Gynecology Service, Virgen de las Nieves University Hospital, 18014 Granada, Spain; [email protected] (M.F.H.-S.); [email protected] (A.P.-P.) 2 Anesthesiology, Resuscitation and Pain Therapy Service, Virgen de las Nieves University Hospital, 18014 Granada, Spain; [email protected] 3 Obstetrics and Gynecology Service, Poniente Hospital, 04700 El Ejido (Almería), Spain; [email protected] 4 Obstetrics and Gynecology Service, Regional University Hospital of Malaga, 29011 Malaga, Spain; [email protected] 5 Department of Obstetrics and Gynecology, University of Granada, 18016 Granada, Spain 6 Consortium for Biomedical Research in Epidemiology & Public Health (CIBER Epidemiología y Salud Pública-CIBERESP), 28029 Madrid, Spain 7 Biohealth Research Institute (Instituto de Investigación Biosanitaria Ibs.GRANADA), 18014 Granada, Spain * Correspondence: [email protected]; Tel.: +34-958242867 Abstract: Background and Objectives: Prematurity is currently a serious public health issue worldwide, because of its high associated morbidity and mortality. Optimizing the management of these preg- nancies is of high priority to improve perinatal outcomes. One tool frequently used to determine the degree of fetal wellbeing is cardiotocography (CTG). A review of the available literature on fetal heart rate (FHR) monitoring in preterm fetuses shows that studies are scarce, and the evidence thus far is unclear. The lack of reference standards for CTG patterns in preterm fetuses can lead to misinterpretation of the changes observed in electronic fetal monitoring (EFM). -

Scalp Eczema Factsheet the Scalp Is an Area of the Body That Can Be Affected by Several Types of Eczema
12 Scalp eczema factsheet The scalp is an area of the body that can be affected by several types of eczema. The scalp may be dry, itchy and scaly in a chronic phase and inflamed (red), weepy and painful in an acute (eczema flare) phase. Aside from eczema, there are a number of reasons why the scalp can become dry and itchy (e.g. psoriasis, fungal infection, ringworm, head lice etc.), so it is wise to get a firm diagnosis if there is uncertainty. Types of eczema • Hair clips and headgear – especially those containing that affect the scalp rubber or nickel. Seborrhoeic eczema (dermatitis) is one of the most See the NES booklet on Contact Dermatitis for more common types of eczema seen on the scalp and hairline. details. It can affect babies (cradle cap), children and adults. The Irritant contact dermatitis is a type of eczema that skin appears red and scaly and there is often dandruff as occurs when the skin’s surface is irritated by a substance well, which can vary in severity. There may also be a rash that causes the skin to become dry, red and itchy. on other parts of the face, such as around the eyebrows, For example, shampoos, mousses, hair gels, hair spray, eyelids and sides of the nose. Seborrhoeic eczema can perm solution and fragrance can all cause irritant contact become infected. See the NES factsheets on Adult dermatitis. See the NES booklet on Contact Dermatitis for Seborrhoeic Dermatitis and Infantile Seborrhoeic more details. Dermatitis and Cradle Cap for more details. -

G Eneral Introduction
Follow-up studies in prenatal medicine Nagel, H.T.C. Citation Nagel, H. T. C. (2007, February 14). Follow-up studies in prenatal medicine. Retrieved from https://hdl.handle.net/1887/9762 Version: Corrected Publisher’s Version Licence agreement concerning inclusion of doctoral thesis in the License: Institutional Repository of the University of Leiden Downloaded from: https://hdl.handle.net/1887/9762 Note: To cite this publication please use the final published version (if applicable). Proefschrift H. Nagel 11-01-2007 13:34 Pagina 11 C H ! T " # $ * eneral introduction 11 Proefschrift H. Nagel 11-01-2007 13:34 Pagina 12 C H ! T " # $ The %etus in prenatal m edicine ccording to M edline de-initions' a conce.tus is an em ,ryo until a .ostconce.tional age o- 9 1 ee2s (or a gestational age o- $* 1 ee2s) and 1 ill then ,e a -etus until ,irth. =- ,orn via,le' the .erson then ,ecom es an in-ant. The -etus is a uni>ue .atient -or several reasons. 6irst' there is a uni>ue relationshi. ,et1 een the m other and her un,orn child. lthough the -etus has his o1 n rights' he or she can only ,e treated via the m other. There-ore the .ur.orted rights o- $ the -etus can never ta2e .recedence over that o- the m other. ;econd' des.ite advances in m edical care there is stri2ing little 2no1 ledge a,out -etal live. ? uestions such as does the -etus [email protected] .ain' does it have a m em ory' are still unans1 ered. -

Prenatal and Preimplantation Genetic Diagnosis for Mps and Related Diseases
PRENATAL AND PREIMPLANTATION GENETIC DIAGNOSIS FOR MPS AND RELATED DISEASES Donna Bernstein, MS Amy Fisher, MS Joyce Fox, MD Families who are concerned about passing on genetic conditions to their children have several options. Two of those options are using prenatal diagnosis and preimplantation genetic diagnosis. Prenatal diagnosis is a method of testing a pregnancy to learn if it is affected with a genetic condition. Preimplantation genetic diagnosis, also called PGD, is a newer technology used to test a fertilized embryo before a pregnancy is established, utilizing in vitro fertilization (IVF). Both methods provide additional reproductive options to parents who are concerned about having a child with a genetic condition. There are two types of prenatal diagnosis; one is called amniocentesis, and the other is called CVS (chorionic villus sampling). Amniocentesis is usually performed between the fifteenth and eighteenth weeks of pregnancy. Amniocentesis involves inserting a fine needle into the uterus through the mother's abdomen and extracting a few tablespoons of amniotic fluid. Skin cells from the fetus are found in the amniotic fluid. These cells contain DNA, which can be tested to see if the fetus carries the same alterations in the genes (called mutations) that cause a genetic condition in an affected family member. If the specific mutation in the affected individual is unknown, it is possible to test the enzyme activity in the cells of the fetus. Although these methods are effective at determining whether a pregnancy is affected or not, they do not generally give information regarding the severity or the course of the condition. -

L&D – Amnioinfusion Guideline and Procedure for Amnioinfusion
L&D – Amnioinfusion Guideline and Procedure for Amnioinfusion. Purpose: Replacing the amniotic fluid with normal saline has been found to be a safe, simple, and very effective way to reduce the occurrence of repetitive variable decelerations. Procedure: Initiation of Amnioinfusion will be ordered and performed by a Certified Nurse Midwife (CNM) or physician (MD). 1. Prepare NS or LR 1000ml with IV tubing in the same fashion as for intravenous infusion. Flush the tubing to clear air. 2. An intrauterine pressure catheter (IUPC) will be placed by the MD/CNM. 3. Elevate the IV bag 3-4 feet above the IUPC tip for rapid infusion. Infuse 250-500ml of solution over a 20-30 minute time frame followed by a 60-180ml/hour maintenance infusion. The total volume infused should not exceed 1000ml unless one has access to ultrasound and can titrate to an amniotic fluid index (AFI) of 8-12 cm to prevent polyhydramnios and hypertonus. 4. If variable decelerations recur or other new non-reassuring FHR patterns develop, notify the MD/CNM. The procedure may be repeated as ordered. 5. Resting tone of the uterus will be increased during infusion but should not increase > 15mmHg from previous baseline. If this occurs, infusion should stop until there is a return to the previous baseline then it can be restarted. An elevated baseline prior to infusion is a contraindication. 6. Monitor for an outflow of infusion. If there is a sudden cessation of outflow fetal head engagement may have occurred increasing the risk of polyhydramnios. Complications are rare but can include iatrogenic polyhydramnios, uterine hypertonus, chorioamnionitis, uterine rupture, placental abruption, and maternal pulmonary embolus. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Proteomic Biomarkers of Intra-Amniotic Inflammation
0031-3998/07/6103-0318 PEDIATRIC RESEARCH Vol. 61, No. 3, 2007 Copyright © 2007 International Pediatric Research Foundation, Inc. Printed in U.S.A. Proteomic Biomarkers of Intra-amniotic Inflammation: Relationship with Funisitis and Early-onset Sepsis in the Premature Neonate CATALIN S. BUHIMSCHI, IRINA A. BUHIMSCHI, SONYA ABDEL-RAZEQ, VICTOR A. ROSENBERG, STEPHEN F. THUNG, GUOMAO ZHAO, ERICA WANG, AND VINEET BHANDARI Department of Obstetrics, Gynecology and Reproductive Sciences [C.S.B., I.A.B., S.A.-R., V.A.R., S.F.T., G.Z., E.W.], and Department of Pediatrics [V.B.], Division of Perinatal Medicine, Yale University School of Medicine, New Haven, CT 06520 ABSTRACT: Our goal was to determine the relationship between 4 vein inflammatory cytokine levels, but not maternal serum val- amniotic fluid (AF) proteomic biomarkers (human neutrophil de- ues, correlate with the presence and severity of the placental fensins 2 and 1, calgranulins C and A) characteristic of intra-amniotic histologic inflammation and umbilical cord vasculitis (7). inflammation, and funisitis and early-onset sepsis in premature neo- Funisitis is characterized by perivascular infiltrates of in- nates. The mass restricted (MR) score was generated from AF flammatory cells and is considered one of the strongest hall- obtained from women in preterm labor (n ϭ 123). The MR score marks of microbial invasion of the amniotic cavity and fetal ranged from 0–4 (none to all biomarkers present). Funisitis was graded histologically and interpreted in relation to the MR scores. inflammatory syndrome (8,9). While there is some debate with Neonates (n ϭ 97) were evaluated for early-onset sepsis. -

Amniotic Fluid Volume: When and How to Take Action
Amniotic fluid volume: When and how to take action http://contemporaryobgyn.modernmedicine.com/pr... Published on Contemporary OB/GYN (http://contemporaryobgyn.modernmedicine.com) Amniotic fluid volume: When and how to take action Alessandro Ghidini, MD and Marta Schilirò, MD and Anna Locatelli, MD Publish Date: JUN 01,2014 Dr. Ghidini is Professor, Georgetown University, Washington, DC, and Director, Perinatal Diagnostic Center, Inova Alexandria Hospital, Alexandria, Virginia. Dr. Schilirò is Medical Doctor, Obstetrics and Gynecology, University of Milano-Bicocca, Monza, Italy. Dr. Locatelli is Associate Professor, University of Milano-Bicocca, Monza, Italy, and Director, Department of Obstetrics and Gynecology, Carate- Giussano Hospital, AO Vimercate-Desio, Italy. None of the authors has a conflict of interest to report with respect to the content of this article. Assessment of amniotic fluid volume (AFV) is an integral part of antenatal ultrasound evaluation during screening exams, targeted anatomy examinations, and in tests assessing fetal well-being. 1 di 17 18/06/2014 11:10 Amniotic fluid volume: When and how to take action http://contemporaryobgyn.modernmedicine.com/pr... Abnormal AFV has been associated with an increased risk of perinatal mortality and several adverse perinatal outcomes, including premature rupture of membranes (PROM), fetal abnormalities, abnormal birth weight, and increased risk of obstetric interventions.1 A recent systematic review demonstrated associations between oligohydramnios, birthweight <10th percentile, and perinatal mortality, as well as between polyhydramnios, birthweight >90th percentile, and perinatal mortality. The predictive ability of AFV alone, however, was generally poor.2 How to assess AFV Ultrasound (U/S) examination is the only practical method of assessing AFV. -
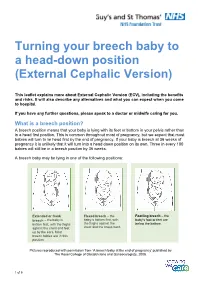
Turning Your Breech Baby to a Head-Down Position (External Cephalic Version)
Turning your breech baby to a head-down position (External Cephalic Version) This leaflet explains more about External Cephalic Version (ECV), including the benefits and risks. It will also describe any alternatives and what you can expect when you come to hospital. If you have any further questions, please speak to a doctor or midwife caring for you. What is a breech position? A breech position means that your baby is lying with its feet or bottom in your pelvis rather than in a head first position. This is common throughout most of pregnancy, but we expect that most babies will turn to lie head first by the end of pregnancy. If your baby is breech at 36 weeks of pregnancy it is unlikely that it will turn into a head down position on its own. Three in every 100 babies will still be in a breech position by 36 weeks. A breech baby may be lying in one of the following positions: Extended or frank Flexed breech – the Footling breech – the breech – the baby is baby is bottom first, with baby’s foot or feet are bottom first, with the thighs the thighs against the below the bottom. against the chest and feet chest and the knees bent. up by the ears. Most breech babies are in this position. Pictures reproduced with permission from ‘A breech baby at the end of pregnancy’ published by The Royal College of Obstetricians and Gynaecologists, 2008. 1 of 5 What causes breech? Breech is more common in women who are expecting twins, or in women who have a differently-shaped womb (uterus). -

318 Reports the Scalp Topography of the Human Visually Evoked Subcortical Potential. G. F. A
Invest. Ophthalmol. Vis. Sci. 318 Reports March 1980 We are indebted to Dr. G. van Lith, Oogziekenhuis, gested the optic nerve as its origin. Cracco and Rotterdam, and to Dr. D. van Norren, Ooglijdersgast- Cracco3 described early oscillatory potentials at huis, Utrecht, for testing our lens design and for sug- 100 cy/sec recorded from a wide scalp distribution gestions for improvement in the manuscript. of electrodes, referred to earlobe electrodes. Early From the Kliniek voor Oogheelkunde, Rijks-Universi- in 1979 we identified a triphasic positive- teit Groningen, Groningen, The Netherlands. Submit- negative-positive component (msec) in some sub- ted for publication July 2, 1979. Reprint requests: Aart jects at latencies of positive 22 (P22)> negative 27 C. Kooijman, Kliniek voor Oogheelkunde, Rijks-Uni- (N27), and positive 35 (Pas).4 Since it appeared im- versiteit Groningen, Oostersingel 59, 9713 EZ Gronin- portant to delineate this component from both the gen, The Netherlands. scalp-recorded ERG and the VECP, we have car- Key words: ERG, Ganzfeld stimulator, LED light ried out a topographic study of the scalp dis- tribution. Materials and method. Observations were made REFERENCES on 14 normal volunteer subjects (eight male and 1. Thijssen JM, Braakhuis W, Pinckers A, and van Lith six female) ages between 19 and 38 years (mean 26 G: Standardized electro-ophthalmography. In Pro- years). All had visual acuities of 6/6 or better. For ceedings of the 170th meeting of the Netherlands this topographical study, electrodes were placed Ophthalmological Society. Junk, The Hague, 1976, according to the International 10/20 system.5 In p. -

Fetal Assement Methods
12/3/2020 Fetal Assement Methods 1 up to 9% of exam 5 to 9 questions 13.00 Adjunct Fetal Surveillance Methods 10%) 13.01 Auscultation (Intermittent Auscultation- IA) 13.02 Fetal movement counting 13.03 Nonstress testing 13.04 Fetal acid base interpretation – will be covered in a separate section 13.05 Biophysical profile 13.06 Fetal Acoustic Stimulation 2 1 12/3/2020 HERE IS ONE FOR YOU!! AWW… Skin to Skin in the OR ☺ 3 Auscultation 4 2 12/3/2020 Benefits of Auscultation • Based on Random Control Trials, neonatal outcomes are comparable to those monitored with EFM • Lower CS rates • Technique is non-invasive • Widespread application is possible • Freedom of movement • Lower cost • Hands on Time and one to one support are facilitated 5 Limitation of Auscultation • Use of the Fetoscope may limit the ability to hear FHR ( obesity, amniotic fluid, pt. movement and uterine contractions) • Certain FHR patterns cannot be detected – variability and some decelerations • Some women may think IA is intrusive • Documentation is not automatic • Potential to increase staff for 1:1 monitoring • Education, practice and skill assessment of staff 6 3 12/3/2020 Auscultation • Non-electronic devices such as a Fetoscope or Stethoscope • No longer common practice in the United States though may be increasing due to patient demand • Allows listening to sounds associated with the opening and closing of ventricular valves via bone conduction • Can hear actual heart sounds 7 Auscultation Fetoscope • A Fetoscope can detect: • FHR baseline • FHR Rhythm • Detect accelerations and decelerations from the baseline • Verify an FHR irregular rhythm • Can clarify double or half counting of EFM • AWHONN, (2015), pp.