Essential Psychopharmacology
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Neural Mechanisms of Oxytocin and Serotonin Interaction in Non-Human Primates and Patients with Autism Arthur Lefevre
Neural mechanisms of oxytocin and serotonin interaction in non-human primates and patients with autism Arthur Lefevre To cite this version: Arthur Lefevre. Neural mechanisms of oxytocin and serotonin interaction in non-human primates and patients with autism. Neuroscience. Université de Lyon, 2016. English. NNT : 2016LYSE1323. tel-01508642 HAL Id: tel-01508642 https://tel.archives-ouvertes.fr/tel-01508642 Submitted on 14 Apr 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N°d’ordre NNT : xxx THESE de DOCTORAT DE L’UNIVERSITE DE LYON opérée au sein de l’Université Claude Bernard Lyon 1 Ecole Doctorale 476 (Neuroscience et Cognition) Spécialité de doctorat : Discipline : Neurosciences Soutenue publiquement le 13 Décembre 2016, par : Arthur LEFEVRE __________________________________________ Neural mechanisms of oxytocin and serotonin interaction in non- human primates and patients with autism __________________________________________ Thèse dirigée par Angela Sirigu (DRCE, CNRS) Devant le jury composé de : GERVAIS Rémi, Président, (Professeur à l’Université Lyon 1 Claude Bernard) CHINI BICE, Rapporteur, (Senior researcher at the Institute of Neuroscience, Milan) CHAKRABARTI Bhismadev, Rapporteur, (Associate Professor at university of Reading and Senior researcher at Cambridge university) KRAUS Christoph, Examinateur, (Senior researcher at the Medical university of Vienna) SIRIGU Angela, Directrice de thèse, (DRCE, Université de Lyon, CNRS) 1 UNIVERSITE CLAUDE BERNARD - LYON 1 Président de l’Université M. -

Dopamine and Serotonin Metabolism in Parkinsonian Models
Dopamine and Serotonin Metabolism in Parkinsonian Models Carmen de la Fuente Barrigon UCL Great Ormond Street Institute of Child Health Thesis submitted for the degree of Doctor of Philosophy (PhD) awarded by University College London (UCL). Funded by Marie Skłodowska-Curie Actions of the European Union’s Seventh Framework Programme (FP7). APRIL 2018 1 I, Carmen de la Fuente Barrigon confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed ………………………………………….. Date ……………………………………………… 3 Dedico esta tesis a los pilares de mi vida. A mis padres, Tomás y Marisa. A mi hermana, Paloma. A mi amor, Francesco. Por vuestros sacrificios, paciencia, amor y apoyo incondicionales que me hacen ser quien soy hoy. 5 Abstract Parkinson’s disease (PD) is a neurodegenerative disorder caused by loss of dopaminergic neurons in the substantia nigra. Different pathogenic mechanisms have been implicated, including loss of mitochondrial complex I function and dysfunction of lysosomal glucocerebrosidase (GBA1) (Neumann et al., 2009; Schapira et al., 1990). Also, it has been hypothesised that serotonin metabolism could be affected in these patients due to the number of enzymes shared by both pathways (Albizu et al., 2011). This thesis considers the potential involvement of complex I and GBA1 in PD using HPLC analysis of changes in the extracellular levels of the metabolites of dopamine and serotonin, and the expression and activity of the enzymes of the dopamine pathway. Using SH-SY5Y cells, complex I deficiency was modelled using rotenone, and GBA1 deficiency was modelled using conduritol B epoxide (CBE). -

4695389.Pdf (3.200Mb)
Non-classical amine recognition evolved in a large clade of olfactory receptors The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Li, Qian, Yaw Tachie-Baffour, Zhikai Liu, Maude W Baldwin, Andrew C Kruse, and Stephen D Liberles. 2015. “Non-classical amine recognition evolved in a large clade of olfactory receptors.” eLife 4 (1): e10441. doi:10.7554/eLife.10441. http://dx.doi.org/10.7554/ eLife.10441. Published Version doi:10.7554/eLife.10441 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:23993622 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA RESEARCH ARTICLE Non-classical amine recognition evolved in a large clade of olfactory receptors Qian Li1, Yaw Tachie-Baffour1, Zhikai Liu1, Maude W Baldwin2, Andrew C Kruse3, Stephen D Liberles1* 1Department of Cell Biology, Harvard Medical School, Boston, United States; 2Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, United States; 3Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, United States Abstract Biogenic amines are important signaling molecules, and the structural basis for their recognition by G Protein-Coupled Receptors (GPCRs) is well understood. Amines are also potent odors, with some activating olfactory trace amine-associated receptors (TAARs). Here, we report that teleost TAARs evolved a new way to recognize amines in a non-classical orientation. -

Neurotransmitters-Drugs Andbrain Function.Pdf
Neurotransmitters, Drugs and Brain Function. Edited by Roy Webster Copyright & 2001 John Wiley & Sons Ltd ISBN: Hardback 0-471-97819-1 Paperback 0-471-98586-4 Electronic 0-470-84657-7 Neurotransmitters, Drugs and Brain Function Neurotransmitters, Drugs and Brain Function. Edited by Roy Webster Copyright & 2001 John Wiley & Sons Ltd ISBN: Hardback 0-471-97819-1 Paperback 0-471-98586-4 Electronic 0-470-84657-7 Neurotransmitters, Drugs and Brain Function Edited by R. A. Webster Department of Pharmacology, University College London, UK JOHN WILEY & SONS, LTD Chichester Á New York Á Weinheim Á Brisbane Á Singapore Á Toronto Neurotransmitters, Drugs and Brain Function. Edited by Roy Webster Copyright & 2001 John Wiley & Sons Ltd ISBN: Hardback 0-471-97819-1 Paperback 0-471-98586-4 Electronic 0-470-84657-7 Copyright # 2001 by John Wiley & Sons Ltd. Bans Lane, Chichester, West Sussex PO19 1UD, UK National 01243 779777 International ++44) 1243 779777 e-mail +for orders and customer service enquiries): [email protected] Visit our Home Page on: http://www.wiley.co.uk or http://www.wiley.com All Rights Reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except under the terms of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London W1P0LP,UK, without the permission in writing of the publisher. Other Wiley Editorial Oces John Wiley & Sons, Inc., 605 Third Avenue, New York, NY 10158-0012, USA WILEY-VCH Verlag GmbH, Pappelallee 3, D-69469 Weinheim, Germany John Wiley & Sons Australia, Ltd. -

Differential Regulation of Mecp2 Phosphorylation in the CNS by Dopamine and Serotonin
Neuropsychopharmacology (2012) 37, 321–337 & 2012 American College of Neuropsychopharmacology. All rights reserved 0893-133X/12 www.neuropsychopharmacology.org Differential Regulation of MeCP2 Phosphorylation in the CNS by Dopamine and Serotonin 1 1 2 1,2,3,4 ,1 Ashley N Hutchinson , Jie V Deng , Dipendra K Aryal , William C Wetsel and Anne E West* 1 2 Department of Neurobiology, Duke University Medical Center, Durham, NC, USA; Department of Psychiatry and Behavioral Sciences, Duke 3 University Medical Center, Durham, NC, USA; Mouse Behavioral and Neuroendocrine Analysis Core Facility, Duke University Medical Center, 4 Durham, NC, USA; Department of Cell Biology, Duke University Medical Center, Durham, NC, USA Systemic administration of amphetamine (AMPH) induces phosphorylation of MeCP2 at Ser421 (pMeCP2) in select populations of neurons in the mesolimbocortical brain regions. Because AMPH simultaneously activates multiple monoamine neurotransmitter systems, here we examined the ability of dopamine (DA), serotonin (5-HT), and norepinephrine (NE) to induce pMeCP2. Selective blockade of the DA transporter (DAT) or the 5-HT transporter (SERT), but not the NE transporter (NET), was sufficient to induce pMeCP2 in the CNS. DAT blockade induced pMeCP2 in the prelimbic cortex (PLC) and nucleus accumbens (NAc), whereas SERT blockade induced pMeCP2 only in the NAc. Administration of selective DA and 5-HT receptor agonists was also sufficient to induce pMeCP2; however, the specific combination of DA and 5-HT receptors activated determined the regional- and cell-type specificity of pMeCP2 induction. The D1-class DA receptor agonist SKF81297 induced pMeCP2 widely; however, coadministration of the D2-class agonist quinpirole restricted the induction of pMeCP2 to GABAergic interneurons of the NAc. -

File Download
Anatomical and functional evidence for trace amines as unique modulators of locomotor function in the mammalian spinal cord Elizabeth A. Gozal, Emory University Brannan E. O'Neill, Emory University Michael A. Sawchuk, Emory University Hong Zhu, Emory University Mallika Halder, Emory University Chou Ching-Chieh , Emory University Shawn Hochman, Emory University Journal Title: Frontiers in Neural Circuits Volume: Volume 8 Publisher: Frontiers | 2014-11-07, Pages 134-134 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.3389/fncir.2014.00134 Permanent URL: https://pid.emory.edu/ark:/25593/mr95r Final published version: http://dx.doi.org/10.3389/fncir.2014.00134 Copyright information: © 2014 Gozal, O'Neill, Sawchuk, Zhu, Halder, Chou and Hochman. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/), which permits distribution of derivative works, making multiple copies, distribution, public display, and publicly performance, provided the original work is properly cited. This license requires credit be given to copyright holder and/or author, copyright and license notices be kept intact. Accessed September 27, 2021 11:18 AM EDT ORIGINAL RESEARCH ARTICLE published: 07 November 2014 NEURAL CIRCUITS doi: 10.3389/fncir.2014.00134 Anatomical and functional evidence for trace amines as unique modulators of locomotor function in the mammalian spinal cord Elizabeth A. Gozal , Brannan E. O’Neill , Michael A. Sawchuk , Hong Zhu , Mallika Halder , Ching-Chieh Chou and Shawn Hochman* Physiology Department, Emory University, Atlanta, GA, USA Edited by: The trace amines (TAs), tryptamine, tyramine, and β-phenylethylamine, are synthesized Brian R. -

Neurophysiological and Neurohumoral Changes by High Frequency Stimulation (HFS) of Nucleus Accumbens (Nac)
From the Department of Neurosurgery of the University of Luebeck Director: Prof. Dr. med. Volker Tronnier Neurophysiological and neurohumoral changes by High Frequency Stimulation (HFS) of Nucleus Accumbens (NAc) Dissertation for Fulfillment of Requirements for the Doctoral Degree of the University of Luebeck from the Department of Natural Sciences Submitted by Ramya Varatharajan Thirugnanasambantham Born in Veeramanipatti-TN, India Luebeck, 2015 I Ramya Varatharajan Thirugnanasambantham Department of Neurosurgery University of Luebeck Ratzeburger allee 160 23562, Luebeck Germany First referee: Prof. Dr. Volker Tronnier Second referee: Prof. Dr. Thomas Martinetz Date of oral examination: 23.09.2015 Approved for printing, Luebeck: 30.09.2015 II Acknowledgements The hard work of so many people is involved in successful completion of this thesis, that a genuine list is virtually impossible. I convey my thanks and gratitude to Prof. Dr. med. Volker Tronnier, Department of Neurosurgery, for providing this opportunity to work under his guidance with this interesting topic. I express my deepest gratitude to Prof. Dr. med. Andreas Moser and Prof. Dr. rer. nat. Ulrich G. Hofmann for providing Laboratory Infrastructure, financial and moral support. Beyond scientific supervision, I was lucky to have the people from liquorlab, guiding me through all matters during my stay in Luebeck. I am thankful to Katharina Schnackenberg whose excellent experience in the laboratory and generous practical help was essential for the successful work at the lab. Thanks to Karin Wiegers, Marie Luis Reher and Gisa Brunk for being like friends and taking good care of me. I am very much thankful to Krishnakumar, Kevin Joseph, Sonya Neto, Mehrnaz Hazrati, Yijing Xie and Susanne Loeffler for their support, encouragement and timely suggestions. -

Imaging Elevated Brain Arachidonic Acid Signaling in Unanesthetized Serotonin Transporter (5-HTT)-Deficient Mice
Neuropsychopharmacology (2009) 34, 1695–1709 & 2009 Nature Publishing Group All rights reserved 0893-133X/09 $32.00 www.neuropsychopharmacology.org Imaging Elevated Brain Arachidonic Acid Signaling in Unanesthetized Serotonin Transporter (5-HTT)-Deficient Mice Mireille Basselin*,1, Meredith A Fox2, Lisa Chang1, Jane M Bell1, Dede Greenstein3, Mei Chen1, 2 1 Dennis L Murphy and Stanley I Rapoport 1Brain Physiology and Metabolism Section, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA; 2Laboratory of Clinical Science, National Institutes of Health, Bethesda, MD, USA; 3Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA Certain polymorphisms reduce serotonin (5-HT) reuptake transporter (5-HTT) function and increase susceptibility to psychiatric +/À disorders. Heterozygous (5-HTT )-deficient mice, models for humans with these polymorphisms, have elevated brain 5-HT concentrations and behavioral abnormalities. As postsynaptic 5-HT2A/2C receptors are coupled to cytosolic phospholipase A2 (cPLA2), which releases arachidonic acid (AA) from membrane phospholipid, 5-HTT-deficient mice may have altered brain AA signaling and metabolism. To test this hypothesis, signaling was imaged as an AA incorporation coefficient k* in unanesthetized homozygous knockout À/À +/À +/+ (5-HTT ), 5-HTT and wild-type (5-HTT ), mice following saline (baseline) or 1.5 mg/kg s.c. DOI, a partial 5-HT2A/2C receptor agonist. Enzyme activities, metabolite concentrations, and head-twitch responses to DOI were also measured. Baseline k* was widely +/À À/À +/+ +/+ elevated by 20–70% in brains of 5-HTT and 5-HTT compared to 5-HTT mice. DOI increased k* in 5-HTT mice, but decreased k* in 5-HTT-deficient mice. -
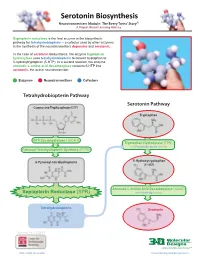
Serotonin Biosynthesis Neurotransmitters Module: the Beery Twins’ Story© a Project-Based Learning Activity
Serotonin Biosynthesis Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity Sepiapterin reductase is the final enzyme in the biosynthetic pathway for tetrahydrobiopterin – a cofactor used by other enzymes in the synthesis of the neurotransmitters dopamine and serotonin. In the case of serotonin biosynthesis, the enzyme tryptophan hydroxylase uses tetrahydrobiopterin to convert tryptophan to 5-hydroxytryptophan (5-HTP). In a second reaction, the enzyme aromatic L-amino acid decarboxylase converts 5-HTP into serotonin, the active neurotransmitter. Enzymes Neurotransmitters Cofactors Tetrahydrobiopterin Pathway Serotonin Pathway Guanosine Triphosphate (GTP) Tryptophan GTP Cyclohydrolase I (GCH1) Tryptophan Hydroxylase (TPH) with Tetrahydrobiopterin Cofactor Pyruvoyl-Tetrahydropterin Synthase (PTPS) 6-Pyruvoyl-tetrahydropterin 5-Hydroxytryptophan (5-HTP) Aromatic L-Amino Acid Decarboxylase (AAAD) Sepiapterin Reductase (SPR) with Vitamin B6 Cofactor Tetrahydrobiopterin Serotonin Version 1.4 -12/2015 ...where molecules become real TM http://cbm.msoe.edu www.3dmoleculardesigns.com Serotonin Biosynthesis Model Guide Neurotransmitters Module: The Beery Twins’ Story© A Project-Based Learning Activity TPH AAAD Tryptophan 5-Hydroxytryptophan Serotonin Tryptophan (Trp or W) is one of the 20 standard 5-Hydroxytryptophan, an intermediate The nal step in the serotonin biosynthesis amino acids and is an essential amino acid that molecule in the serotonin biosynthesis pathway requires the removal of the cannot be synthesized by the human body. pathway, is formed by the addition of a carboxylic acid group (COOH) from the Tryptophan is composed of the standard amino hydroxyl (OH) group to the fth carbon of the backbone of 5-hydroxytryptophan to form acid backbone with an indole ring side chain. indole ring of tryptophan. -

Receptor Interaction Profiles of 4-Alkoxy-Substituted 2,5-Dimethoxyphenethylamines and Related Amphetamines
ORIGINAL RESEARCH published: 28 November 2019 doi: 10.3389/fphar.2019.01423 Receptor Interaction Profiles of 4-Alkoxy-Substituted 2,5-Dimethoxyphenethylamines and Related Amphetamines Karolina E. Kolaczynska 1, Dino Luethi 1,2, Daniel Trachsel 3, Marius C. Hoener 4 and Matthias E. Liechti 1* 1 Division of Clinical Pharmacology and Toxicology, Department of Biomedicine, University Hospital Basel and University of Basel, Basel, Switzerland, 2 Center for Physiology and Pharmacology, Institute of Pharmacology, Medical University of Vienna, Vienna, Austria, 3 ReseaChem GmbH, Burgdorf, Switzerland, 4 Neuroscience Research, pRED, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland Background: 2,4,5-Trimethoxyamphetamine (TMA-2) is a potent psychedelic compound. Structurally related 4-alkyloxy-substituted 2,5-dimethoxyamphetamines and phenethylamine congeners (2C-O derivatives) have been described but their pharmacology Edited by: is mostly undefined. Therefore, we examined receptor binding and activation profiles of M. Foster Olive, these derivatives at monoamine receptors and transporters. Arizona State University, United States Methods: Receptor binding affinities were determined at the serotonergic 5-HT1A, 5-HT2A, Reviewed by: Luc Maroteaux, and 5-HT2C receptors, trace amine-associated receptor 1 (TAAR1), adrenergic α1 and INSERM U839 Institut du Fer à α2 receptors, dopaminergic D2 receptor, and at monoamine transporters, using target- Moulin, France Simon D. Brandt, transfected cells. Additionally, activation of 5-HT2A and 5-HT2B receptors and TAAR1 was Liverpool John Moores University, determined. Furthermore, we assessed monoamine transporter inhibition. United Kingdom Results: Both the phenethylamine and amphetamine derivatives (Ki = 8–1700 nM and *Correspondence: Matthias E. Liechti 61–4400 nM, respectively) bound with moderate to high affinities to the 5-HT2A receptor [email protected] with preference over the 5-HT1A and 5-HT2C receptors (5-HT2A/5-HT1A = 1.4–333 and Specialty section: 5-HT2A/5-HT2C = 2.1–14, respectively). -

Modulation of Serotonin Signaling/Metabolism by Akkermansia Muciniphila and Its Extracellular Vesicles Through the Gut-Brain
www.nature.com/scientificreports OPEN Modulation of serotonin signaling/ metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut‑brain axis in mice Rezvan Yaghoubfar1,2, Ava Behrouzi1,2, Fatemeh Ashrafan1,2, Arefeh Shahryari1,2, Hamid Reza Moradi3, Samira Choopani4, Shima Hadifar1,2, Farzam Vaziri1,2, Seyed Ali Nojoumi1,2, Abolfazl Fateh1,2*, Shohreh Khatami 5* & Seyed Davar Siadat1,2 Several studies have reported that the host‑microbe interactions in the gut modulate the host serotonin or 5‑hydroxytryptamine (5‑HT) system. Here, we evaluated the efects of Akkermansia muciniphila and its extracellular vesicles (EVs) on genes pertaining to the serotonergic system in the colon and hippocampus of mice. Male C57BL/6J mice were administered viable A. muciniphila and its EVs for 4 weeks. The serotonin levels in the colon, hippocampus, and serum of mice, as well as the human colon carcinoma cells (Caco‑2), were measured by ELISA assays. Also, the efects of A. muciniphila and its EVs on the expression of serotonin system genes in the colon and hippocampus were examined. A. muciniphila and its EVs may have a biological efect on the induction of serotonin levels in the colon and hippocampus of mice. Also, EVs increased the serotonin level in the Caco‑2 cell line. In contrast, both treatments decreased the serotonin level in the serum. Both the bacterium and its EVs had signifcant efects on the mRNA expression of genes, involved in serotonin signaling/ metabolism in the colon and hippocampus of mice. Moreover, A. muciniphila and its EVs afected the mRNA expression of infammatory cytokines (Il-10 and Tnf‑α) in the colon, however, there is no signifcant diference in infammatory cell infltrate in the histopathology of the colon. -

Serotonin Pathway in Cancer
International Journal of Molecular Sciences Review Serotonin Pathway in Cancer Pragathi Balakrishna 1, Sagila George 1, Hassan Hatoum 1,* and Sarbajit Mukherjee 2,* 1 Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; [email protected] (P.B.); [email protected] (S.G.) 2 Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA * Correspondence: [email protected] (H.H.); [email protected] (S.M.) Abstract: Serotonin (5-hydroxytryptamine, 5-HT) is a biogenic monoamine produced from the essen- tial amino acid tryptophan. Serotonin’s role as a neurotransmitter in the central nervous system and a motility mediator in the gastrointestinal tract has been well defined, and its function in tumorigenesis in various cancers (gliomas, carcinoids, and carcinomas) is being studied. Many studies have shown a potential stimulatory effect of serotonin on cancer cell proliferation, invasion, dissemination, and tumor angiogenesis. Although the underlying mechanism is complex, it is proposed that serotonin levels in the tumor and its interaction with specific receptor subtypes are associated with disease progression. This review article describes serotonin’s role in cancer pathogenesis and the utility of the serotonin pathway as a potential therapeutic target in cancer treatment. Octreotide, an inhibitor of serotonin release, is used in well-differentiated neuroendocrine cancers, and the tryptophan hy- droxylase (TPH) inhibitor, telotristat, is currently being investigated in clinical trials to treat patients with metastatic neuroendocrine tumors and advanced cholangiocarcinoma. Several in vitro studies have shown the anticancer effect of 5-HT receptor antagonists in various cancers such as prostate cancer, breast cancer, urinary bladder, colorectal cancer, carcinoid, and small-cell lung cancer.